In this article, I briefly explain the peptides and proteins with their distinguishing properties.
Proteins
Proteins are the most abundant biological macromolecules present in all cells in many varieties. In a single cell, thousands of different kinds of proteins are present. Peptides and proteins are the polymers of amino acids.
In every organism, the proteins are built from the same twenty amino acids. Amino acids are covalently linked in a particular linear sequence to produce a specific protein.
The same twenty amino acids combine in many different combinations and sequences eventually, providing the ability to cells to produce proteins with remarkably different properties. Among the diverse protein products, the enzymes are the specialized ones.
Peptides
Peptides are chains of amino acids as two amino acid molecules are covalently linked through an amide linkage known as a peptide bond. A peptide bond is formed by the removal of one water molecule when two amino acids are linked with each other (Figure).
Peptides can be dipeptides, tripeptides, or tetrapeptides according to the number of amino acids linked with each other. An oligopeptide is a chain of few amino acids, whereas a polypeptide is a chain of many amino acids. There is a molecular weight difference between proteins and polypeptides. Proteins have a molecular weight of more than 10,000, whereas the latter has less than 10,000 molecular weight.
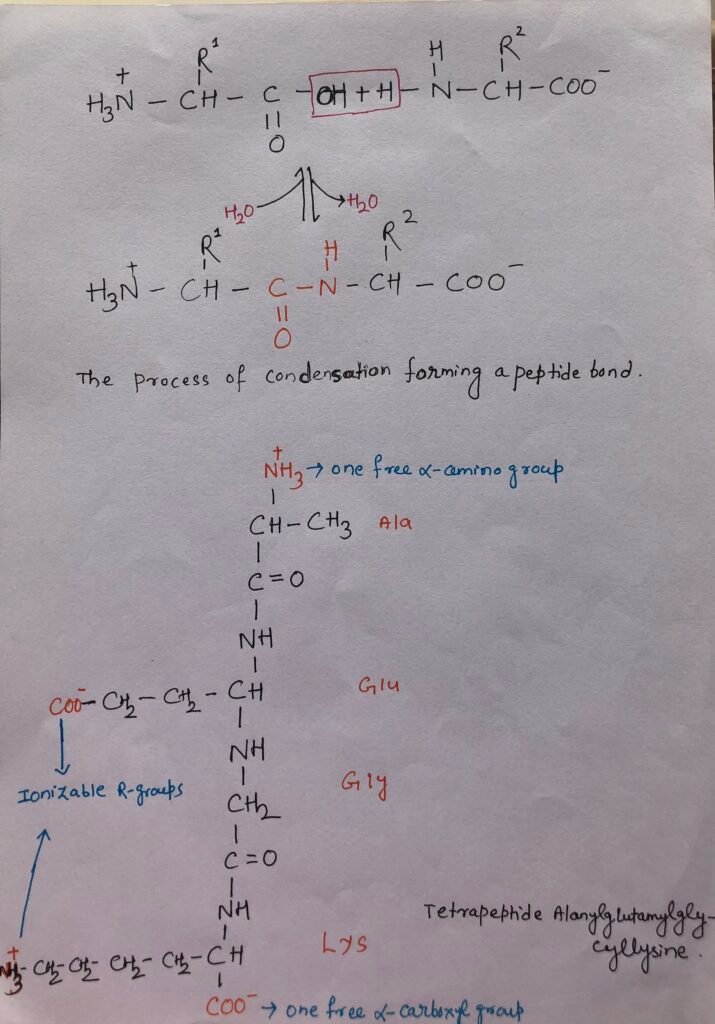
In a peptide, polypeptide, or protein, the sequence of amino acids shows that on the left, the amino-terminal end is placed, whereas the right end includes the carboxy-terminal moiety.
Only one free α-amino group and one free α-carboxyl group present at the opposite ends of a peptide chain ionize. Whereas the other non-terminal α-amino groups, α-carboxyl groups of other amino acids don’t ionize. Thus, this does not contribute to the total acid-base behavior of peptides.
Some amino acids possess ionizable R groups, which contribute to the total acid-base properties of the molecule (Figure). So, the acid-base behavior of a peptide can be known from its free α-amino group and α-carboxyl groups, including the number and nature of its ionizable R groups. Peptides have characteristic titration curves and a characteristic isoelectric pH where they do not move in an electric field.
Peptides and proteins vary in molecular weight and their different effects
The function of biologically active peptides and proteins is independent of their molecular weights. The smallest peptides have marked biological effects, e.g., L-aspartyl-L-phenylalanine methyl ester (aspartame) is commercially synthesized as an artificial sweetener.
Peptides can range in length from two to many thousands of amino acid residues. Smaller peptides, at even a low concentration, show characteristic effects.
Several vertebrate hormones, like oxytocin, which is only nine amino acids long, are secreted by the posterior pituitary gland. It stimulates the contraction of the uterus. The hypothalamus produces thyrotropin-releasing factor, which consists of only three amino acids and stimulates the release of thyrotropin hormone from the anterior pituitary gland.
Variations in proteins
In proteins, the length of polypeptide chains varies considerably. The majority of naturally occurring proteins have fewer than 2000 amino acid residues. However, some proteins do have more than 2000 amino acid residues.
Lysozyme, a protein present in chicken egg white, has 129 amino acid residues in a single chain with a molecular weight of 14,300. Human protein cytochrome c, has 104 amino acids linked in one chain with a molecular weight of 12,400.
Human hemoglobin has 574 amino acid residues linked in four polypeptide chains, whereas human serum albumin has 609 amino acid residues in a single polypeptide chain. The human muscle protein titin has 26,926 amino acid residues in a single polypeptide chain with a molecular weight of about 2,993,000.
Some proteins with higher amino acid residues and having more than one polypeptide chain are given in the table below.
| Protein | No.of residues | Molecular weight | Polypeptide chains |
| Titin (human) | 26926 | 2993000 | 1 |
| Glutamine synthetase (E. coli) | 5628 | 619000 | 12 |
| Apolipoprotein B (Human) | 4536 | 513000 | 1 |
| RNA polymerase (E.coli) | 4158 | 450000 | 5 |
| Hexokinase (yeast) | 972 | 107900 | 1 |
| Hemoglobin (human) | 574 | 64500 | 4 |
Multisubunit proteins
Multisubunit proteins consist of more than one identical or different noncovalently associated polypeptide chains.
If a multisubunit protein consists of more than two polypeptide chains, among which two chains are identical then the protein is said to be an oligomeric protein, and the identical units are known as protomers.
Hemoglobin in humans has four polypeptide chains, among which, two are identical alpha chains and two are identical beta chains. All four polypeptide chains are associated with non-covalent interactions. Hemoglobin is a dimer of αβ protomers or a tetramer of four polypeptide subunits.
Insulin is among some proteins, which has two polypeptide chains linked covalently by disulfide bonds.
Proteins vary with variable amino acid composition. A protein does not consist of all the twenty amino acids in equal amounts. It may contain one amino acid once or more than once or may be devoid of a particular amino acid.
Determination of number of amino acid residues and molecular weight of an amino acid residue in protein
In a simple protein, the approximate number of amino acid residues can be determined by dividing its molecular weight by 110. The average molecular weight of an amino acid residue in a protein can be determined by subtracting the molecular weight of a water molecule from the average molecular weight of protein amino acids, which is 128.
So, the average molecular weight of an amino acid residue in a protein is 128-18 = 110. A molecule of water is removed to form a peptide bond.
Simple proteins and conjugated proteins
Proteins containing only amino acid residues are known as simple proteins. The enzymes chymotrypsin and ribonuclease A are simple proteins that upon hydrolysis, yield only amino acids.
However, some proteins contain amino acids along with some other chemical components and are called conjugated proteins.
The component other than amino acid is called its prosthetic group, e.g., in lipoproteins, lipids are present as prosthetic groups. Conjugated proteins can be named or classified according to the nature of their prosthetic group. The prosthetic group may be of metal or organic, e.g., glycoproteins contain sugar prosthetic groups, whereas metalloproteins contain precise metal prosthetic groups.
Some conjugated proteins are listed in the table below.
| Classification | Prosthetic group | Example |
| Phosphoproteins | Phosphate | Casein of milk |
| Hemoproteins | Heme | Hemoglobin |
| Metalloproteins | Zinc Iron Copper Calcium | Alcohol dehydrogenase Ferritin Plastocyanin Calmodulin |
| glycoproteins | Carbohydrates | IgG (immunoglobulin G) |
| Flavoproteins | Flavin nucleotides | Succinate dehydrogenase |
Conclusion
Peptides and proteins are the polymers of amino acids. Peptides and proteins vary with distinguishing properties. The same twenty amino acids combine in many different combinations and sequences eventually, providing the ability to cells to produce proteins with remarkably different properties.
Peptides are chains of amino acids. Two amino acid molecules are covalently linked through an amide linkage known as a peptide bond. Peptides and proteins vary in their molecular weight and functions.
Simple proteins are proteins with only amino acid residues, whereas conjugated proteins contain amino acids along with some other chemical components. The component other than amino acid is called its prosthetic group.
You may also like:
- Electrophoresis: The process of separation of proteins
- Breakthrough treatments of HIV based on the protease mechanism
- Allosteric enzymes
- Proteins facilitate the transbilayer movement of a lipid molecule

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.
