In this article, I briefly describe the breakthrough treatments of the human immunodeficiency virus (HIV) based on the protease mechanism.
The disease caused by HIV and the symptoms
The human immunodeficiency virus (HIV) attacks our immune system. The acquired immunodeficiency syndrome, a chronic immune system disease, is caused by HIV. It lowers the immunity of our body. Thus, the body loses its capacity to fight infectious diseases.
The symptoms of the disease can appear in the form of fever, fatigue, and swollen lymph nodes. HIV multiplies and destroys immune cells. If left untreated, HIV can be converted into AIDS within 8-10 years of the introduction of the virus into the body. HIV can be within control with proper medications, which do not allow the disease to progress.
The mechanism of serine protease chymotrypsin
There are different kinds of specific enzyme reaction mechanisms. The protease is an enzyme that catalyzes the hydrolytic cleavage of peptide bonds. The bovine pancreatic chymotrypsin specifically catalyzes the cleavage of peptide bonds adjacent to aromatic amino acid (Trp, Phe, Tyr) residues.
With the presence of the enzyme chymotrypsin, the rate of hydrolysis of peptide bonds can be elevated by a factor of 109. Rather than catalyzing a direct attack on water, the enzyme forms a transient covalent acyl-enzyme intermediate. Thus, the reaction takes place in two distinct phases, i.e., the acylation and the de-acylation phase.
The acylation phase includes the cleavage of the peptide bond and the formation of an ester linkage between the peptide carbonyl carbon and the enzyme. The de-acylation phase includes the hydrolysis of the ester linkage and regeneration of the nonacylated enzyme.
Chymotrypsin, apart from acting on polypeptides, also catalyzes the hydrolysis of small esters and amides. The process of hydrolysis is much slower compared to peptide hydrolysis because of the availability of less binding energy with smaller substrates.
Researchers’ view
According to researchers B.A. Kilby and B.S. Hartley, the hydrolysis of the ester P-nitrophenyl acetate is measured by the release of p-nitrophenol, which proceeds with a rapid burst and comes down to a slower rate afterward.
Hartley and Kilby concluded that the burst phase correlated with the release of just under one molecule of p-nitrophenol for the presence of every enzyme molecule. The researchers suggested that the acylation phase involves the release of p-nitrophenol. It’s because there is a rapid acylation of all the enzyme molecules followed by a slower de-acylation phase.
The protease mechanism leads to the development of new treatments for HIV infections
The human immunodeficiency virus (HIV) is the causative agent of the disease, acquired immune deficiency syndrome (AIDS). In the year 1980s, AIDS was first recognized as a worldwide epidemic. Around 34 to 41 million HIV-infected people were living in the world in the year 2015. In addition to that, around 2 million newly infected people were added to the number, with approximately 1.2 million fatalities were also happened in that year.
Structure of HIV
The HIV was identified as a retrovirus, having an RNA genome with a reverse transcriptase enzyme (figure 1). It inserts a DNA copy of its RNA genome into the DNA of the host cell. Thus, it starts its invasion, ultimately changing the genome of the host cell. The structure of HIV is roughly spherical, about 120nm in diameter. It consists of two copies of single-stranded RNA that codes for nine viral genes enclosed in a conical capsid (figure 2).
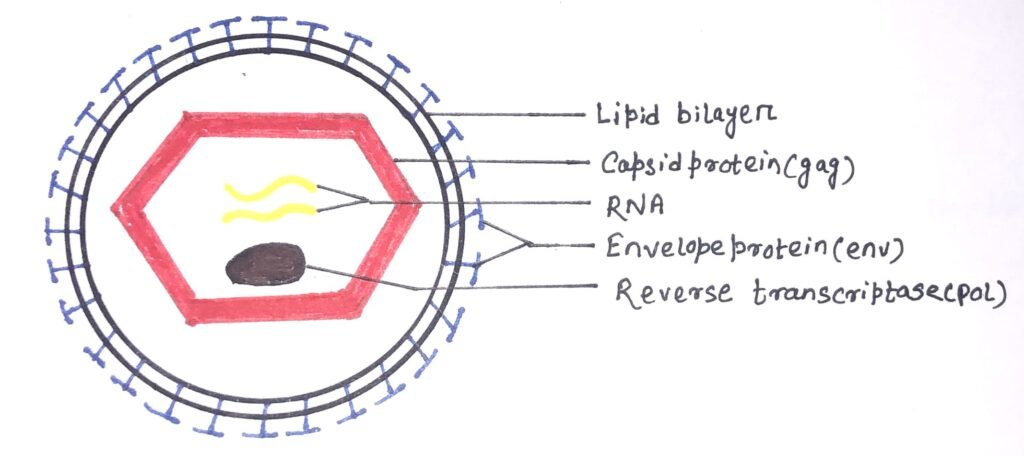
The single-stranded RNA has a tight attachment with the nucleocapsid proteins, p7 viral protein, and the necessary enzymes for virion development. The required enzymes for virion development are reverse transcriptase, proteases, ribonuclease, and integrase. The integrity of the virion particle is ensured by a matrix surrounding the capsid.
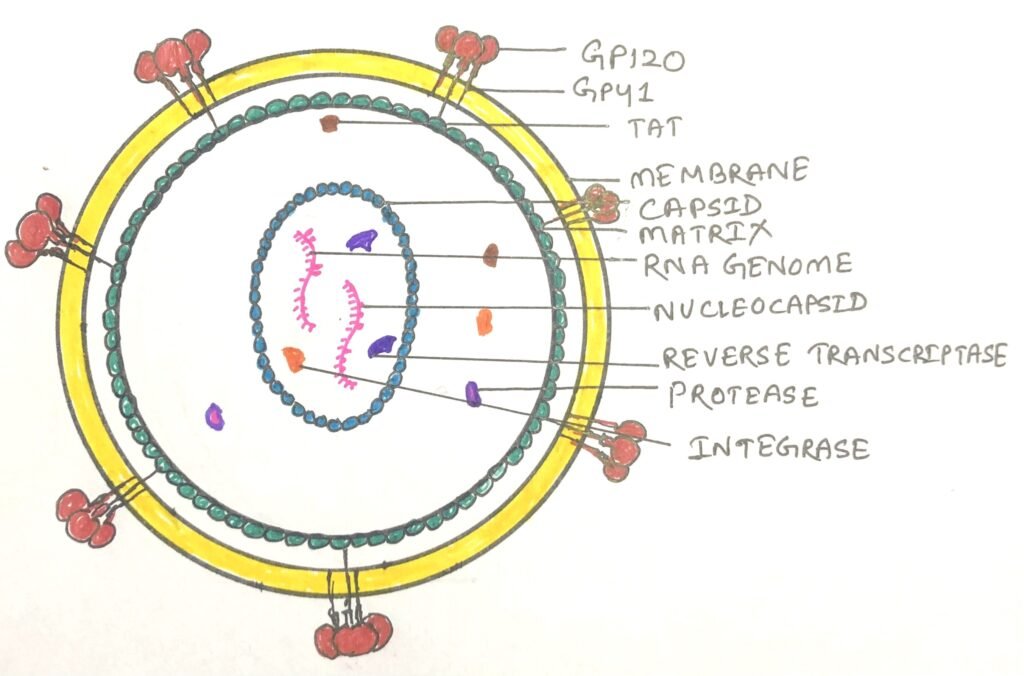
Structure and function of the retrovirus
The viral envelope is composed of a lipid bilayer surrounding the matrix (figure 1). The envelope contains host cell proteins with a few copies of the HIV envelope protein. The HIV env gene encodes the envelope protein, which helps the virus to attach to target cells. It also fuses the target cell’s membrane with the viral envelope. Thus, it releases the viral contents into the host cell and initiates the infectious cycle. The HIV vaccine efforts mainly target the viral envelope protein, as it is the only surface protein of the virus.
A retrovirus has an uncomplicated life cycle where it converts its RNA genome into duplex DNA in many steps that are catalyzed by the enzyme reverse transcriptase. Then, it inserts its duplex DNA into the host cell by the action of an integrase enzyme.
Within the host cell, the integrated copy of the viral genome stays at a dormant stage for an indefinite period. It can also be transcribed back into RNA, which can be translated into proteins to prepare new virus particles. Generally, the viral genes are translated into large polyproteins. An enzyme HIV protease, cuts them into individual proteins that take part in the making of the virus.
The protease mechanism
New pharmaceutical agents are designed to inhibit an enzyme. Three enzymes, the reverse transcriptase, the integrase, and the protease, play a vital role in the life cycle of HIV. These enzymes are the most promising drug targets in the treatment of HIV infection.
The proteases are present in four major subclasses, i.e., serine proteases, cysteine proteases, aspartyl proteases, and metalloproteases. The serine proteases, chymotrypsin proteases, trypsin proteases, and cysteine proteases form covalent enzyme-substrate complexes. The aspartyl proteases and metalloproteases do not form covalent enzyme-substrate complexes.
The HIV protease- aspartyl protease
The human immunodeficiency virus protease is an aspartyl protease in which two active site Asp residues ease the direct attack of a water molecule on the carbonyl group of the peptide bond to be cleaved. As a result, the initial product, an unstable tetrahedral intermediate like in the chymotrypsin reaction is formed.
This tetrahedral intermediate is similar to the structure of the reaction transition state. The HIV protease inhibitor drugs form noncovalent complexes with the enzyme. The interaction is a very tight one, and thus, the protease inhibitors are known as irreversible inhibitors. The tight binding is a result of the design of the drugs, analogous to the transition state of the enzyme.
The HIV protease efficiently cleaves peptide bonds between phenylalanine and proline residues. The enzyme’s active site has a pocket that binds an aromatic group present next to the bond to be cleaved. Different HIV protease inhibitors differ in their structure. However, all possess a core having a main chain with a hydroxyl group positioned adjacent to the benzyl group in a branch.
This type of arrangement targets the benzyl group to an aromatic binding pocket. In the tetrahedral intermediate, the adjacent hydroxyl group mimics the negatively charged oxygen in the normal reaction. Thus, it provides a transition state analog. Each inhibitor is designed such that its remainder fits and binds to different types of clefts along the enzyme’s surface. Thus, it increases the overall binding affinity.
These effective drugs are influencing the lives of HIV-infected people positively. Their availability has increased the quality and lifestyle of affected people. At the beginning of the year 2015, about 15 million people of 37 million people were undergoing antiretroviral therapy.
Conclusion
The deadly disease AIDS is caused by HIV, a retrovirus having an RNA genome with a reverse transcriptase enzyme. The breakthrough treatments of HIV are based upon the protease mechanism. The three enzymes, i.e., the reverse transcriptase, the integrase, and the protease, play a vital role in the life cycle of HIV. These enzymes are used as the most promising drug targets in the treatment of AIDS.
These effective drugs are influencing the lives of HIV-infected people positively. Their availability has increased the quality and lifestyle of affected people.
You may also like:

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around twelve years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.
