In this article, I briefly describe type-II hypersensitivity with its effects.
Hypersensitivity
The inflammatory reactions within the humoral or cell-mediated branches of the immune system, causing extensive tissue damage or occasionally death, are known as hypersensitive reactions. The reactions are immediate or may be delayed type depending on the symptoms.
In 1963, P.G.H. Gell and Robin Coombs categorized hypersensitive reactions into four types. The four types of reactions are named type-I, type-II, type-III, and type-IV hypersensitive reactions. The type-I, type-II, and type-III hypersensitive reactions are mediated by antibody or antigen-antibody complexes within the humoral branch of the immune system. The type-IV hypersensitivity is a delayed-type hypersensitivity, which occurs within the cell-mediated branch of the immune system. Immediate hypersensitivity includes symptoms that manifest within minutes or hours after a sensitized recipient encounters an antigen. Type-4 hypersensitivity includes the delayed onset of symptoms after antigen exposure, also called delayed-type hypersensitivity.
Type-II hypersensitive reaction
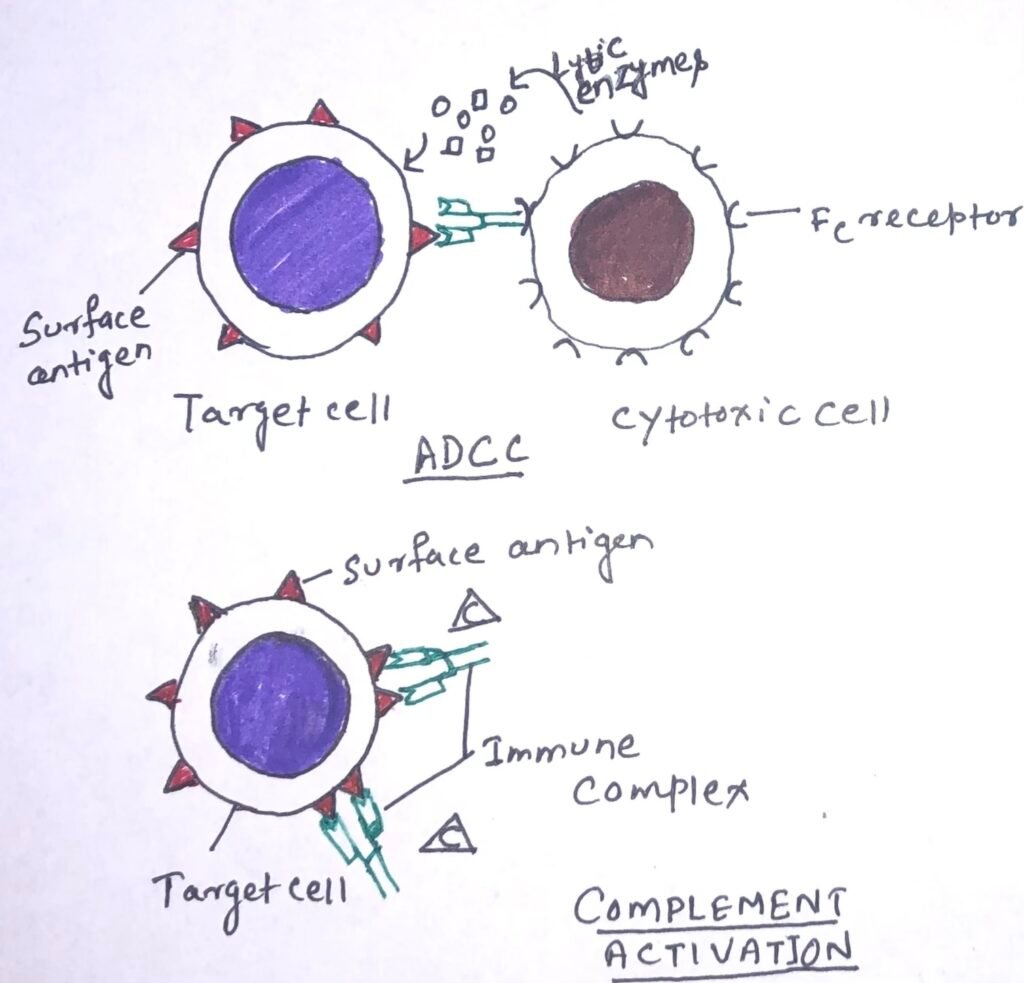
Type-II hypersensitive reactions involve antibody-mediated destruction of cells. When an antibody reacts with antigenic determinants present on the surface of cells, it leads to cell damage or death through complement-mediated lysis or antibody-dependent cell-mediated cytotoxicity (ADCC). Transfusion reactions and hemolytic disease of the newborn are type-II hypersensitive reactions.
Antibody-dependent cell-mediated cytotoxicity (ADCC)
It is a mechanism of cell-mediated immune defense where cytotoxic cells bearing Fc receptors bind to the Fc region of antibodies on target cells and promote the killing of the cells. Several cells having cytotoxic potential express membrane receptors for the Fc region of the antibody molecule. When the antibody is specifically bound to a target cell, these receptor-bearing cells can bind to the antibody Fc region and so to the target cells, which subsequently causes lysis of the target cell by releasing lytic enzymes (Figure 1). The cells that mediate ADCC are NK cells, macrophages, monocytes, neutrophils, and eosinophils.
Transfusion reaction
A hemolytic transfusion reaction can occur after a blood transfusion. It is a serious complication reaction and occurs when the red blood cells that were given during the transfusion are destroyed by the person’s immune system. A large number of proteins and glycoproteins on the membrane of red blood cells are encoded by different genes. Each gene has several alternative alleles. A person having one allelic form of a blood group antigen can recognize other allelic forms on transfused blood as foreign and mount an antibody response.
Blood types are classified as A, B, or O, and the surface antigens associated with the blood types are identified as A, B, and H, respectively. The A, B, and H antigens are synthesized by a series of enzymatic reactions catalyzed by glycosyltransferases. Antigens of the ABO blood type initially detected on the surface of red blood cells. However, they also occur on the surface of other cells, as well as in antibody secretions.
Iso hemagglutinins and transfusion reaction
Antibodies to the A, B, and O antigens, called iso hemagglutinins, are usually of the IgM class. Iso hemagglutinins are normally found within the human population. Most adults have IgM antibodies to those members of the ABH family they do not express. It is because microorganisms express carbohydrate antigens similar in structure to the carbohydrates of the ABH system and induce a B-cell response. The host expresses B cell-producing antibodies specific for the ABH antigens and undergoes negative selection. An example of this is an individual with blood type A recognizes B-like epitopes on microorganisms and generates iso hemagglutinins to the B-like epitopes. The individual does not respond to A-like epitopes on the same microorganisms as they have developed toleration to self-A epitopes.
When a blood type A individual is transfused to blood containing type B cells, a transfusion reaction occurs. In this process, the anti-B iso hemagglutinins bind to the B blood cells and destroy them through complement-mediated lysis. The clinical manifestations of transfusion reactions result from massive intravascular hemolysis of the transfused RBCs by another plus complement. These manifestations may be either immediate or delayed.
Acute hemolytic transfusion reaction
It is a type of transfusion reaction that is associated with hemolysis and occurs immediately after the transfusion within 24 hours post-transfusion. The reaction is also known as an “immediate hemolytic transfusion reaction”. It is a medical emergency, which results from the rapid destruction of the donor red blood cells by host antibodies (IgG, IgM).
In ABO blood group incompatibility – the most severe one involves the transfer of group A red cells to a patient with group O type blood. The typical symptoms include fever, chills, nausea, clotting within blood vessels, pain in the lower back, and hemoglobin in the urine. There are two types of acute hemolytic transfusion reactions, i.e., immune-mediated and non-immune-mediated. The immune-mediated reaction is caused by preformed IgM anti-A, anti-B, or both, which results in severe intravascular hemolysis. The damaged RBCs before transfusion result in a non-immune mediated reaction, leading to hemoglobinemia and hemoglobinuria. The occurrence of free hemoglobin is detected in the plasma within hours. It is filtered through the kidneys, resulting in hemoglobinuria and the presence of excessive free hemoglobin in the blood.
Delayed hemolytic transfusion reaction
It is developed in between 2 to 6 days after transfusion and is called a delayed hemolytic transfusion reaction. Generally, it occurs in individuals who have received repeated transfusions of ABO-compatible blood that is incompatible with other blood group antigens. IgG antibodies are produced against a variety of blood groups of membrane antigens, such as Rh, Kidd, Kell, and Duffy. This is induced by the transfused blood. IgG antibodies are less effective than IgM in activating complement. Thus, complement-mediated lysis of the transfused red blood cells remains incomplete, and many of the transfused cells are destroyed at extravascular sites by agglutination, opsonization, and subsequent phagocytosis by macrophages.
Free hemoglobin is usually not detected in the plasma or urine in these reactions. The symptoms of this include fever, low hemoglobin, increased bilirubin, mild jaundice, and anemia. When the hemoglobin is degraded, the porphyrin component is metabolized to bilirubin. The presence of bilirubin at high levels is toxic to the organism and results in the disease bilirubinemia. The symptoms of the disease are fever, chills, nausea, clotting within blood vessels, pain in the lower back, and hemoglobin in the urine. The treatment includes the quick termination of transfusion, and urine flow is maintained with a diuretic.
Hemolytic disease of the newborn (HDN)- A type-II reaction
It is a disease of the newborn that develops when maternal IgG antibodies specific for fetal blood-group antigens cross the placenta, leading to the destruction of fetal red blood cells. The effects of such transfer vary from minor, serious to even lethal. Hemolytic disease of the newborn (HDN) used to be a major cause of fetal loss and death among newborn babies.
During pregnancy, some of the mother’s antibodies are transported across the placenta and enter the fetal circulation. This is necessary because newborns have only a primitive immune system at the time of birth. The continuous presence of maternal antibodies helps ensure that they survive while their immune system matures.
However, when maternal IgG antibodies specific for fetal blood-group antigens cross the placenta, fetal red blood cells undergo destruction. This leads the newborn to suffer from hemolytic disease. When the mother and fetus express different alleles of the Rhesus (Rh) antigen, severe hemolytic disease of the newborn, called erythroblastosis fetalis, develops. There are five alleles of the Rh antigen, i.e., c, C, D, E, and e. However, the expression of the D allele elicits the strongest immune response. Individuals having the D allele of the Rh antigen are designated as Rh+.
Pregnancies at risk of hemolytic disease of the newborn (HDN) are those in which an RhD-negative mother becomes pregnant with an RhD-positive child (the child has inherited the D antigen from the father). The mother’s immune system responds to the fetal D antigen by forming antibodies (IgG antibodies) against it (anti-D).
Production of Rh-specific plasma cells and memory B cells in mother
When a woman is pregnant, the fetal red blood cells are separated from the mother’s circulation by a layer of cells in the placenta called the trophoblast. During her first pregnancy with an Rh+ fetus, an Rh– woman is usually not exposed to enough fetal red blood cells to activate her Rh-specific B cells. But at the time of delivery, the placenta separates from the uterine wall. This allows large amounts of fetal umbilical cord blood to enter the mother’s circulation.
These fetal red blood cells activate Rh-specific B cells. This results in the production of Rh-specific plasma cells and memory B cells in the mother (Figure 2). The secreted IgM antibody (Figure 2) clears the Rh+ fetal red cells from the mother’s circulation. However, the remaining memory cells get activated in a subsequent pregnancy with an Rh+ fetus, which results in the formation of IgG anti-RhD antibodies. These antibodies cross the placenta and damage the fetal red blood cells (Figure 2).

Consequences of HDN and its detection
The nature of HDN is mild, moderate, or severe depending on the rate of hemolysis. At birth, mild symptoms include mild anemia and jaundice, both of which may be cured without any treatment. The conversion of hemoglobin to bilirubin can bring an added threat to the newborn. This is because the lipid-soluble bilirubin may accumulate in the brain and cause brain damage. Very young babies can suffer fatal brain damage from bilirubin. However, bilirubin is rapidly broken down upon exposure of the skin to UV light. Babies having significantly high levels of blood bilirubin are treated by exposure of the skin to UV light in their cribs.
Maternal serum is tested at intervals during pregnancy for antibodies to the Rh antigen. This helps in the detection of the development of hemolytic disease in the newborn. In vivo, these antibodies destroy Rh D-positive fetal RBCs. However, in vitro, they do not lyse cells or even cause agglutination, making them difficult to identify. The Coombs test, named after Robin Coombs, helps to detect the presence of maternal IgG on the surface of fetal red blood cells. Robin Coombs first developed the technique of using antibodies that are targeted against other antibodies.
Prevention and treatment of HDN
Hemolytic disease of the newborn (HDN) caused by Rh incompatibility can be entirely prevented in a subsequent pregnancy by administering antibodies against the Rh antigen to the mother at around 28 weeks of her first pregnancy and within 24-48 hours after the first delivery. These antibodies, called RhoGAM, bind to any fetal red blood cells that may have entered the mother’s circulation at the time of delivery. These facilitate their clearance before B-cell activation and ensuing memory cell production can take place. In a subsequent pregnancy with an Rh+ fetus, a mother who has been administered RhoGAM is unlikely to produce IgG anti-Rh antibodies. Thus, protecting the fetus from the damage that would occur when these antibodies cross the placenta.
If hemolytic disease caused by Rh incompatibility is detected during pregnancy, the pregnancy is carefully monitored for the severity of HDN. Regular ultrasound scans of the fetus are done, and the amount of anti-D in the mother’s serum is monitored. A rise in anti-D is an indicator of active hemolysis. If a fetal blood test confirms severe fetal anemia, the fetus can be given an intrauterine blood exchange transfusion to replace fetal Rh+ red blood cells with Rh– cells. These transfusions are given every 10-21 days until delivery.
However, if the severity is less, a blood exchange transfusion is not given until after birth. The infant is also exposed to low levels of UV light to break down the bilirubin, thus preventing cerebral damage. The mother can also undergo a treatment during the pregnancy by plasmapheresis. It is a procedure, which involves a cell separation machine separating the mother’s blood into two fractions, i.e., cells and plasma. The plasma containing the anti-Rh antibody is discarded. Then the cells are reinfused into the mother in an albumin or fresh plasma solution.
Hemolytic disease of the newborn by ABO blood group incompatibility
Hemolytic disease of the newborn can also be caused by an incompatibility of the ABO blood group. Nearly around 65% of HDNs, are caused by ABO blood-group incompatibility between the mother and the fetus. These are not severe reactions. It arises when a mother with blood type O becomes pregnant with a fetus with a different blood type (type A, B, or AB).
The mother’s serum contains naturally occurring anti-A and anti-B, which tend to be of the IgG class. Therefore, it can cross the placenta in subsequent pregnancies and cause the hemolysis of fetal RBCs. The ABO incompatibility HDN is usually less severe when compared to Rh incompatibility. Generally, the fetal anemia resulting from ABO incompatibility is mild. The major clinical manifestation includes a slight elevation of bilirubin, with jaundice. The treatment involves exposing the infant to low levels of UV light. This leads to the breakdown of the bilirubin and avoids cerebral damage. The risk of sensitization to the Rh D antigen is decreased if the fetus is ABO incompatible. This is because any fetal cells that leak into the maternal circulation are quickly demolished by potent maternal anti-A and/or anti-B, minimizing the likelihood of maternal exposure to the D antigen.
Drug-induced hemolytic anemia- A type-II disorder
Drug-induced immune hemolytic anemia is a blood disorder. It happens when a medicine triggers the body’s immune system to attack its red blood cells. As a result, red blood cells undergo hemolysis. Certain antibiotics, such as penicillin, cephalosporin, and streptomycin, along with other drugs like ibuprofen and naproxen, can adsorb non-specifically to proteins on RBC membranes. Thus, it forms a drug-protein complex similar to a hapten-carrier complex.
In some patients, such drug-protein complexes induce the formation of antibodies. These antibodies when bound to the adsorbed drug on red blood cells, induce complement-mediated lysis, leading to progressive anemia. The symptoms of the disease may include dark urine, fatigue, pale skin color, rapid heart rate, shortness of breath, and jaundice. When the drug is stopped further, the anemia symptoms begin to disappear.
Conclusion
Hypersensitivity reactions are the inflammatory reactions within the humoral or cell-mediated branches of the immune system. These reactions cause extensive tissue damage, or occasionally death. The four types of reactions are named type-I, type-II, type-III, and type-IV hypersensitive reactions.
Type-II hypersensitive reactions involve antibody-mediated destruction of cells. Transfusion reactions and hemolytic disease of the newborn are type-II hypersensitive reactions. A hemolytic transfusion reaction can occur after a blood transfusion. It is a serious complicated reaction and occurs when the red blood cells that were given during the transfusion are destroyed by the person’s immune system. It is of two types, i.e., acute hemolytic transfusion reaction and delayed hemolytic transfusion reaction.
Hemolytic disease of the newborn develops when maternal IgG antibodies specific for fetal blood-group antigens cross the placenta, leading to the destruction of fetal red blood cells. HDN caused by Rh incompatibility can be entirely prevented in a subsequent pregnancy by administering antibodies against the Rh antigen to the mother at around 28 weeks of her first pregnancy and within 24-48 hours after the first delivery. Hemolytic disease of the newborn can also be caused by an incompatibility of the ABO blood group. It arises when a mother with blood type O becomes pregnant with a fetus with a different blood type (type A, B, or AB).
Drug-induced hemolytic anemia is a type II disorder, which develops when a medicine triggers the body’s immune system to attack its red blood cells. Certain antibiotics, such as penicillin, cephalosporin, and streptomycin, along with other drugs like ibuprofen and naproxen, can adsorb non-specifically to proteins on RBC membranes, thus forming a drug-protein complex. These drug-protein complexes induce antibody formation. Inhibiting the intake of the drug can diminish the disease symptoms.
You may also like:
- Type-I hypersensitive reaction
- The delayed-type hypersensitivity: a cell-mediated response
- Type-III Hypersensitivity

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around twelve years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.
