In this article, I briefly describe the role of Fc receptors in antibody effector functions. Fc receptors (FcRs) are specialized cell-surface receptors that bind to the Fc region of antibodies and link humoral immunity with cellular immune responses. By interacting with different antibody classes, FcRs regulate key immune functions, such as phagocytosis, cytotoxicity, inflammation, and immune homeostasis.
Fc Receptors
Antibodies are highly versatile molecules of the immune system that protect the body against pathogens, their toxins, and abnormal cells arising from infection or malignant transformation. They exert their protective effects through multiple mechanisms. While certain antibody classes can directly damage pathogens by activating the complement system and inducing cell lysis, many antibody functions rely on interactions with specific receptors present on immune and non-immune cells.
These receptors, known as Fc receptors (FcRs), were identified over four decades ago and are so named because they bind specifically to the Fc (constant) region of antibodies. Fc receptors are expressed on cells of both the innate and adaptive immune systems, as well as on epithelial and endothelial cells, enabling antibodies to influence a wide range of immune responses.
By engaging Fc receptors, antibodies transfer their antigen specificity to otherwise non-specific immune cells, allowing these cells to direct their effector activities precisely toward antibody-coated targets. In this way, Fc receptors form a crucial functional link between humoral immunity and cell-mediated immune responses. Under resting conditions, Fc receptors generally do not transmit signals when bound to antibodies alone. However, clustering of Fc receptors by multiple antibody–antigen complexes triggers intracellular signaling pathways that initiate effector functions such as phagocytosis, cytokine release, or cell-mediated cytotoxicity.
Importantly, not all Fc receptors activate immune responses. Some Fc receptors deliver inhibitory signals that restrain cellular activation. Individual immune cells often express both activating and inhibitory Fc receptors, and the overall cellular response depends on the balance between these opposing signals following engagement with antibody–antigen complexes.
FcR Signaling
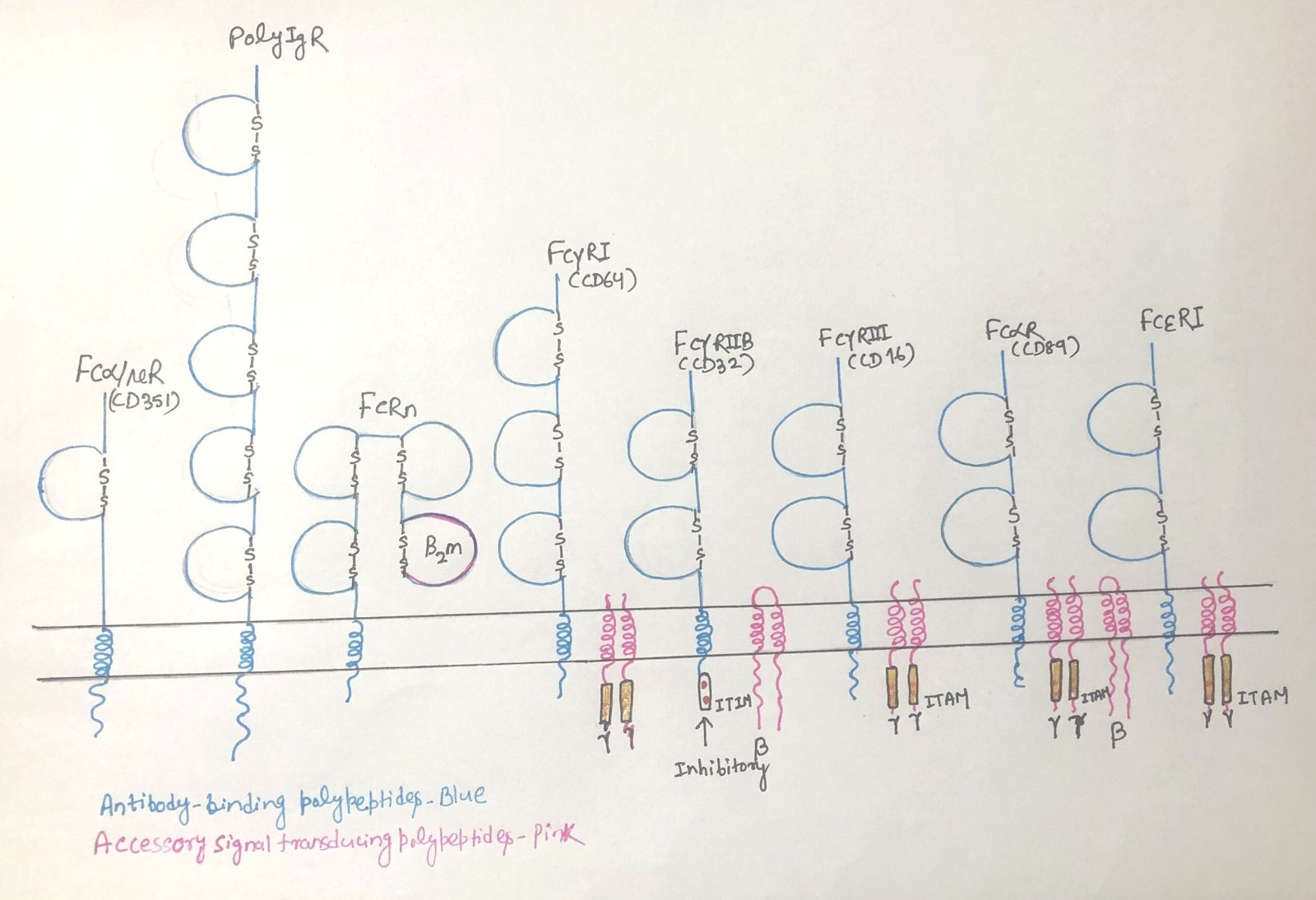
Fc receptors must be cross-linked by multiple antibodies bound to a single target to trigger a response. This clustering generates signals that either activate or inhibit the cell’s effector functions. The nature of the signal depends on the receptor’s association with specific motifs; activating receptors link to ITAMs (immunoreceptor tyrosine-based activation motifs). Whereas inhibitory receptors associate with ITIMs (immunoreceptor tyrosine-based inhibition motifs). Some activating Fc receptors contain ITAMs within their own structure, whereas others rely on a shared gamma chain to provide the activating signal (Figure 1).
ITAM-Mediated Fc Receptor Signaling and Cellular Responses
Immunoreceptor tyrosine-based activation motifs (ITAMs) are conserved signaling sequences (Figure 1) widely present in immune system proteins. They are located in the cytoplasmic regions of receptor-associated molecules involved in antigen recognition, such as the CD3 complex of T cell receptors (TCRs) and the Igα–Igβ heterodimer of B cell receptors (BCRs). Upon receptor engagement, ITAMs become targets for tyrosine kinases, initiating intracellular signaling cascades.
Fc receptor–associated ITAM signaling closely resembles that of TCRs and BCRs. Activation of these pathways leads to the recruitment of additional kinases, phosphorylation of downstream signaling molecules, and production of key second messengers such as diacylglycerol (DAG) and calcium ions. Together, these signals drive the activation of cells expressing Fc receptors.
Despite these shared signaling mechanisms, the functional outcome of Fc receptor activation varies depending on the receptor type, the signaling proteins present, and the specific cell involved. In macrophages, Fc receptor signaling enhances phagocytosis and promotes cytokine production. Due to this signaling, granulocytes such as eosinophils, mast cells, and basophils degranulate and release inflammatory mediators, often contributing to allergic reactions. In natural killer (NK) cells, engagement of Fcγ receptors with IgG-coated target cells initiates antibody-dependent cellular cytotoxicity (ADCC), leading to apoptosis of the target cell.
ITIM-Containing Fc Receptors and Immune Regulation
Unlike activating Fc receptors, the inhibitory Fc receptor FcγRIIB (Figure 1) contains an immunoreceptor tyrosine-based inhibitory motif (ITIM) in its cytoplasmic domain rather than an ITAM. FcγRIIB is the principal inhibitory receptor within the Fc receptor family. ITIM motifs are also present in other immune regulatory molecules, such as the B-cell surface protein CD22, which helps terminate B-cell receptor (BCR) signaling once sufficient antibody production has occurred and the pathogen has been cleared.
ITIMs function by recruiting phosphatases instead of kinases. These enzymes, including SHIP (SH2-containing inositol phosphatase), counteract activating signals by removing phosphate groups from key signaling molecules, thereby dampening intracellular signaling pathways.
FcγRIIB plays a crucial role in setting the activation threshold of immune cells. FcγRIIB binds multiple IgG subclasses, and several cell types, particularly B cells and dendritic cells, express it. When antibody–antigen complexes engage FcγRIIB on B cells, the receptor delivers inhibitory signals through the B-cell receptor. It suppresses further activation and indicates that dendritic cells accumulate sufficient antibody levels. IgG-coated antigens engage FcγRIIB and limit cell activation and maturation. This prevents unintended responses to low levels of circulating immune complexes. Full dendritic cell activation, therefore, requires additional strong signals, such as those delivered through Toll-like receptors (TLRs) and other pattern-recognition receptors.
Many immune cells express both activating and inhibitory Fc receptors, allowing them to finely adjust their responses based on the balance of opposing signals. The immune environment influences the pattern of Fc receptor expression. Cytokines such as TGF-β and IL-4 promote expression of inhibitory Fc receptors, whereas inflammatory mediators, including TNF and IFN-γ, enhance the expression of activating Fc receptors.
Fcγ Receptors and IgG-Mediated Effector Functions
Fcγ receptors represent the most diverse subgroup of Fc receptors and are key mediators of antibody-dependent immune responses. A wide variety of cells express these receptors, and they specifically recognize IgG antibodies, often binding multiple IgG subclasses. Most Fcγ receptors are activating in nature and play major roles in immune defense.
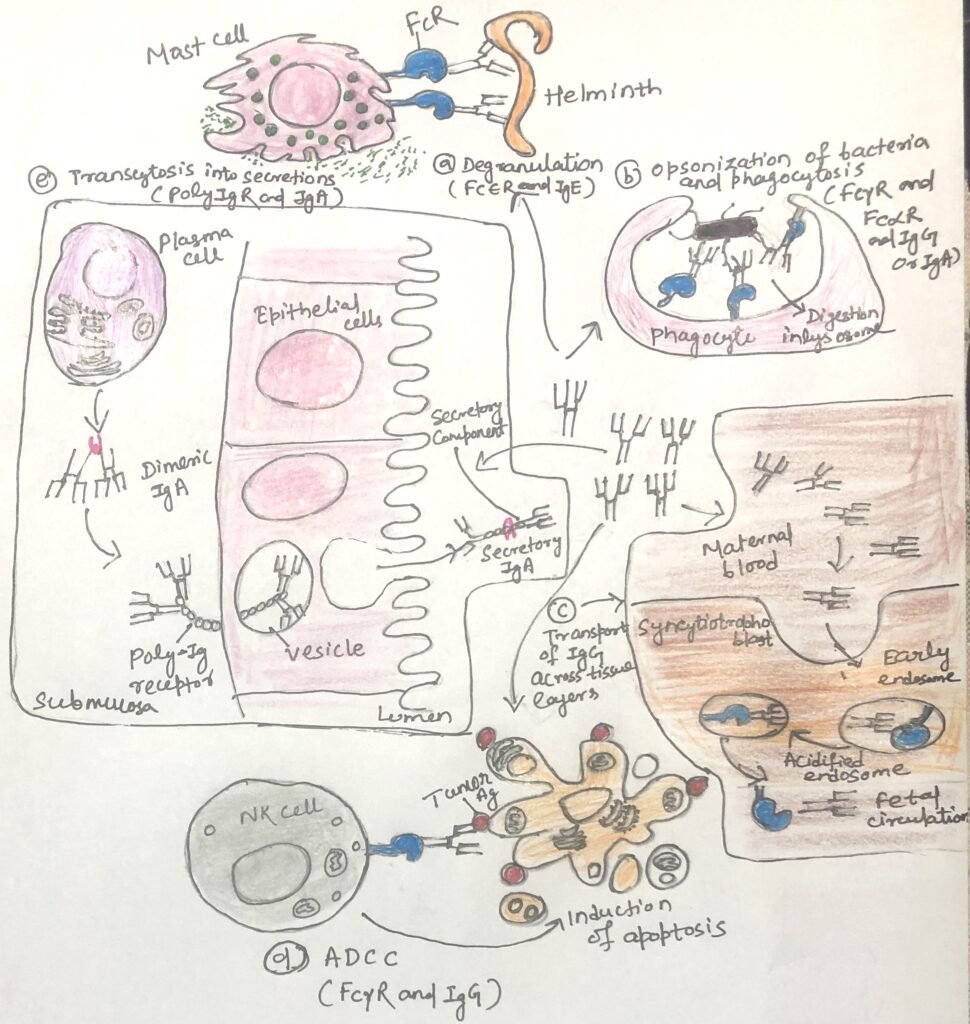
When expressed on monocytes, macrophages, and dendritic cells, activating Fcγ receptors triggers phagocytosis of IgG-coated targets (Figure 2b). This process leads to antigen breakdown and presentation of antigen-derived peptides on MHC class II molecules, thereby linking innate and adaptive immunity. On granulocytes, Fcγ receptor engagement induces degranulation and release of inflammatory mediators. In natural killer (NK) cells, Fcγ receptors recognize antibodies bound to target cells and initiate antibody-dependent cell-mediated cytotoxicity (ADCC) (Fig. 2d).
Four Fcγ receptor families, FcγRI (CD64), FcγRII (CD32), FcγRIII (CD16), and FcγRIV, are conserved among mammals. Of these, FcγRI, FcγRIII, and FcγRIV function as activating receptors and differ in their affinity for IgG and their patterns of cellular expression. FcγRI exhibits high-affinity binding and preferentially recognizes IgG2a in mice and IgG1 and IgG3 in humans, whereas FcγRIII binds IgG with lower affinity. Most immune cells, excluding mature T cells, express both FcγRI and FcγRIII, while NK cells express FcγRIII exclusively.
Within the FcγRII family, FcγRIIA functions as an activating receptor, whereas FcγRIIB (CD32) serves as the principal inhibitory Fcγ receptor. Except for mature T and NK cells, most immune cells express the receptor FcγRIIB. Upon binding antibody–antigen complexes, it delivers inhibitory signals that raise the activation threshold of the cell, thereby limiting excessive responses by B cells, dendritic cells, and macrophages.
FcεR (Fcε Receptors) and IgE-Mediated Immune Responses
Fcε receptors (FcεR) mediate the effects of IgE antibodies and are central to allergic reactions and defense against parasites. The immune system recognizes two types of Fcε receptors. Mast cells, basophils, and eosinophils express the high-affinity FcεRI, while B cells and eosinophils express the low-affinity FcεRII (CD23), a C-type lectin.
FcεRI (Figure 1) binds circulating IgE with high affinity, allowing the antibodies to remain attached even in the absence of antigen. When multivalent antigens—such as parasites or allergens—cross-link IgE on FcεRI, strong activating signals trigger cell degranulation (Figure 2a). This releases mediators like histamine, proteases, and cytokines, which help eliminate parasites but also drive the clinical symptoms of allergies.
In contrast, FcεRII binds IgE more weakly and primarily regulates IgE levels and modulates immune responses rather than directly triggering degranulation. Together, these receptors link IgE specificity to potent cellular effector mechanisms, orchestrating both protective and pathological immune responses.
Fcα Receptor (FcαR/CD89) and IgA-Mediated Immunity
The Fcα receptor (FcαR/CD89) (figure 1) is expressed on myeloid cells, including monocytes, macrophages, granulocytes, and dendritic cells. As an activating Fc receptor, FcαR triggers immune effector functions upon cross-linking by IgA-containing immune complexes (Fig. 2b). This engagement promotes phagocytosis, antibody-dependent cellular cytotoxicity (ADCC), degranulation, cytokine release, and production of reactive oxygen species, all of which help eliminate pathogens.
Interestingly, monomeric IgA in the circulation can deliver inhibitory signals through FcαR, limiting excessive inflammation and preventing tissue damage that might result from widespread Fc receptor activation. This dual ability allows FcαR to balance pathogen clearance with immune regulation.
Fcμ Receptors and IgM-Dependent Immune Functions
B cells, T cells, and natural killer (NK) cells express the Fcμ receptor (FcμR), which regulates cellular activation. In addition to FcμR, another IgM-binding receptor, Fcα/μR, recognizes IgM with high affinity and IgA with moderate affinity. B cells, macrophages, and follicular dendritic cells express this receptor. Engagement of Fcα/μR by IgM-coated antigens promotes phagocytosis, contributing to early immune defense mechanisms.
Polymeric Immunoglobulin Receptor (polyIgR) and Mucosal Immunity
Epithelial cells express the polymeric immunoglobulin receptor (polyIgR). It serves a specialized role in immune defense. PolyIgR mediates the transport of polymeric IgA and IgM across epithelial barriers, moving these antibodies from the underlying tissues and bloodstream into the lumens of mucosal and glandular sites. These sites include the gastrointestinal, respiratory, and reproductive tracts, as well as salivary, lacrimal, and mammary glands.
Among the antibodies transported by polyIgR, IgA predominates, becoming the principal immunoglobulin present in mucosal secretions. By delivering IgA to these exposed surfaces, polyIgR provides essential immune protection at body entry points, where the risk of pathogen exposure is highest.
Neonatal Fc Receptor and Passive IgG Immunity
The neonatal Fc receptor (FcRn) is a specialized Fcγ receptor with a unique structure and function. FcRn is structurally related to MHC class I molecules and associates with β2-microglobulin. Many cell types, including epithelial and vascular endothelial cells, express the receptor. It binds both IgG and albumin, protecting them from degradation and thereby extending their half-lives in circulation.
FcRn plays a critical role in maternal and neonatal immunity. In the placenta, it transports IgG from the mother to the fetus, providing passive immune protection before birth. After birth, FcRn mediates the transfer of IgG from breast milk across the infant’s intestinal epithelium into the bloodstream, offering continued protection while the infant’s immune system matures. Although its expression decreases after weaning, FcRn continues to support mucosal immunity by transporting antibody–antigen complexes to immune tissues for antigen processing. In addition, FcRn can complement the function of polyIgR by contributing to antibody transport at mucosal surfaces and can promote pathogen uptake and destruction when expressed on phagocytic cells.
Conclusion
Fc receptors serve as critical molecular links between antibody specificity and cellular immune responses. By recognizing different antibody classes, Fc receptors translate antigen–antibody binding into a wide range of effector functions, including phagocytosis, cytotoxicity, degranulation, cytokine release, and immune regulation. The balance between activating and inhibitory Fc receptors, mediated through ITAM and ITIM signaling pathways, allows immune cells to finely tune their responses and prevent excessive inflammation. Specialized Fc receptors such as FcεR, FcαR, FcμR, polyIgR, and FcRn further extend antibody function by regulating allergic responses, mucosal immunity, early immune defense, antibody transport, and passive protection of the fetus and newborn. Together, these diverse Fc receptor systems ensure that antibodies provide effective protection while maintaining immune homeostasis.
You may also like:

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.