In this article, I briefly describe the mechanism of killing of target cells by effector cells.
Effector cells
Effector cells are the cells of the adaptive immune system, carrying out an immune function in response to a stimulus. The group of effector cells that have direct cytotoxic activity on foreign cells and altered self-cells are known as cytotoxic effector cells. These cells can be classified into two groups. The first group includes the antigen-specific cytotoxic T lymphocytes (CTLs), and the second group includes the nonspecific cells, i.e., the NK cells and macrophages. The target cells to which these effector cells are directed can be listed as allogeneic, malignant, virus-infected, and chemically conjugated cells. The sub-population of effector CD4+ T cells is another group of effector cells, which mediates delayed-type hypersensitivity reactions.
The mechanism of killing of the target cell by CTLs
The mechanism of cell killing by a CTL begins when it first makes direct contact with its target cell through the T cell receptor (TCR) and class I MHC molecules, respectively. The TCR-CD3 membrane complex on a CTL recognizes an antigen in association with class I MHC molecule on a target cell. This antigen-specific recognition leads to the binding of the integrin receptor LFA-1 on the CTL membrane to the ICAMs (intracellular cell adhesion molecules) on the target cell membrane. This binding results in the formation of a conjugate.
The cell-adhesion molecules on the surface of T cells, i.e., CD2 and integrin LFA-1, bind to LFA-3 and ICAMs, respectively, present on the antigen-presenting cells and various target cells. These cell-adhesion molecules facilitate TCR-mediated interactions.
The action of perforins and granzymes
The fully differentiated cytotoxic lymphocytes carry many granules containing perforin and granzymes. After the formation of a CTL-target cell conjugate, the Golgi stacks and storage granules reorient within the cytoplasm of the CTL and get concentrated near the junction with the target cell. The interaction between CTL and the target cell raises the intracellular Ca2+ concentration. The rising intracellular Ca2+ concentration causes the release of the perforin monomers and granzyme proteases from granules by exocytosis into the junctional space between the two cells. When the perforin monomers make contact with the target cell, the monomers get polymerized by perforin polymerase enzymes to form cylindrical pores in the target cell membrane (Figure 1).

The serine proteases and granzymes enter the target cell through the channels and activate caspases and nucleases in the target cell, resulting in apoptosis. CTLs can kill the target cell either by the perforin/granzyme pathway or through the Fas pathway. Some potent CTLs lacking perforin and granzymes mediate cytotoxicity by Fas, a transmembrane protein. The protein can deliver a death signal during the crosslinking with its natural ligand, the Fas ligand. The interaction of the Fas ligand found on the membrane of CTLs, with Fas present on a target cell, begins apoptosis, the process of cell death.
The perforin/granzyme pathway
In the perforin-assisted pathway, perforin helps granzyme to kill the target cell. Generally, the target cells possess mannose-6-phosphate receptors on their surface. Many target cells possess a receptor known as mannose-6-phosphate on their surface. This receptor binds with granzyme B, and the binding complex is internalized into the vesicles of the target cell. Perforin releases granzyme B from the vesicles into the cytoplasm of the target cell. Granzyme initiates a cascade of reactions in the cytoplasm, which leads to the death of the target cell.
The granzymes introduced into the target cell activate an initiator caspase. Caspase incorporates the elements cysteine, aspartate, and protease. Caspases are present in the cell as inactive proenzymes, called procaspases, which convert into an active initiator caspase by the proteolytic cleavage. An active initiator caspase cleaves other procaspases, thus activating their proteolytic activity. The activation of a caspase cascade results in the apoptotic death of the cell.
The Fas pathway
A member of the TNF-receptor family, Fas is a transmembrane protein and is found on the target cell membrane. The Fas ligand is a member of the tumor necrosis family crosslinks Fas. It is found on the membrane of cytotoxic lymphocytes. Fas interacts with the FAS ligand, and this interaction activates an initiator caspase in the target cell. Fas is associated with a protein known as Fas-associated protein with a death domain (FADD). The FADD, in turn, associates with a procaspase form of caspase 8. When the Fas ligand crosslinks Fas, the procaspase 8 gets activated and gets converted into caspase 8. Then, it initiates an apoptotic caspase cascade, which ultimately leads to cell death.
The mechanism of killing of target cell by NK cell
Natural killer (NK) cells are bone marrow-derived lymphocytes, possess a large granular morphology, and provide immune defenses against viruses and tumors. The NK cells can efficiently kill target cells in an antibody-independent manner. These cells appear to kill tumor cells and virus-infected cells similarly to that of the cytotoxic T lymphocytes. However, NK cells and CTLs differ from each other in some way.
The natural killer cells are cytotoxic as they possess granules containing perforin and granzyme in their cytoplasm. When they interact with a target cell, degranulation with the release of perforin and granzymes takes place at the junction of interaction. NK cells kill the Fas-bearing target cells through the Fas ligand present on their surface. NK cells don’t express antigen-specific receptors like cytotoxic T cells. Further, antigen recognition by NK cells is not MHC-restricted. The response by NK cells does not generate any immunologic memory.
The signal receptors from NK cells
NK cells receive two types of signals from two different receptors. One type of receptor gives inhibition signals, whereas another one gives activation signals to NK cells. Some of the activation receptors belong to the class of carbohydrate-binding proteins known as C-type lectins. NKR-P1 is a C-type lectin found on NK cells that possess activation properties. The other molecules, i.e., CD2 and CD16 present in NK cells, might be involved in activation. The activation of human NK cells depends upon the three additional proteins, i.e., Nkp30, Nkp44, and Nkp46, which play a significant role.
Interaction between inhibitory and activating signals
NK cells possess two major groups of inhibitory receptors, which together are referred to as the inhibitory-receptor superfamily (IRS). The two groups can be distinguished, i.e., a family of C-type-lectin-inhibitory receptors (CLIR) and the other is a group of Ig-superfamily-inhibitory receptors (ISIR), also known as the killer-cell-inhibitory receptors (KIR).
NK cells when activated, can mediate direct cytotoxicity or secrete cytokines and chemokine that modulate subsequent steps in the adaptive immune response. The combination of signals from activating and inhibitory receptors regulates these functions. The MHC class I receptors are particularly important for NK cells to discriminate “self” (healthy cells) from “altered self” (infected- and transformed cells).
The interactions between the MHC class I receptor and ligand can induce inhibitory signals to NK cells that counteract activating receptor signals, which results in NK cell inhibition. In contrast, loss of MHC class I expression during viral infection or carcinogenesis leads to NK cell activation and target cell destruction by removing this inhibitory signal.
The two main families of inhibitory receptors involved in NK regulation by MHC class I are the Killer cell inhibitory receptors (KIR) and the C-type lectin-like CD94/NKG2A heterodimers. The CD94/NKG2 receptors recognize HLA-E on potential target cells, whereas KIR interacts with the classical MHC class Ia (HLA-A, -B, and -C). These receptors are expressed in a combinatorial fashion on NK cells to generate an NK cell repertoire.
The restricted cytotoxic activity of NK cells towards altered self-cells
NK cells have activating receptors that interact with the activating receptor ligands (AR ligands) present in the target cells. These receptor ligands may consist of abnormal patterns of glycosylation on the surface of virus-infected or transformed cells. The activating receptors after recognizing these determinants, send signals to NK cells to kill the target cells (Figure 2).
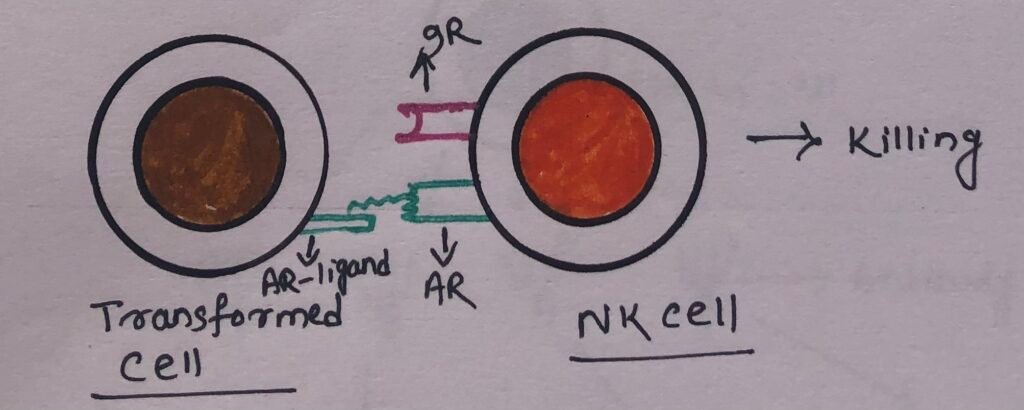
However, the engagement of inhibitory NK cell receptors such as KIR and CD94/NKG2 by class I MHC molecules on potential target cells delivers a signal that stops the activation signal (Figure 3).
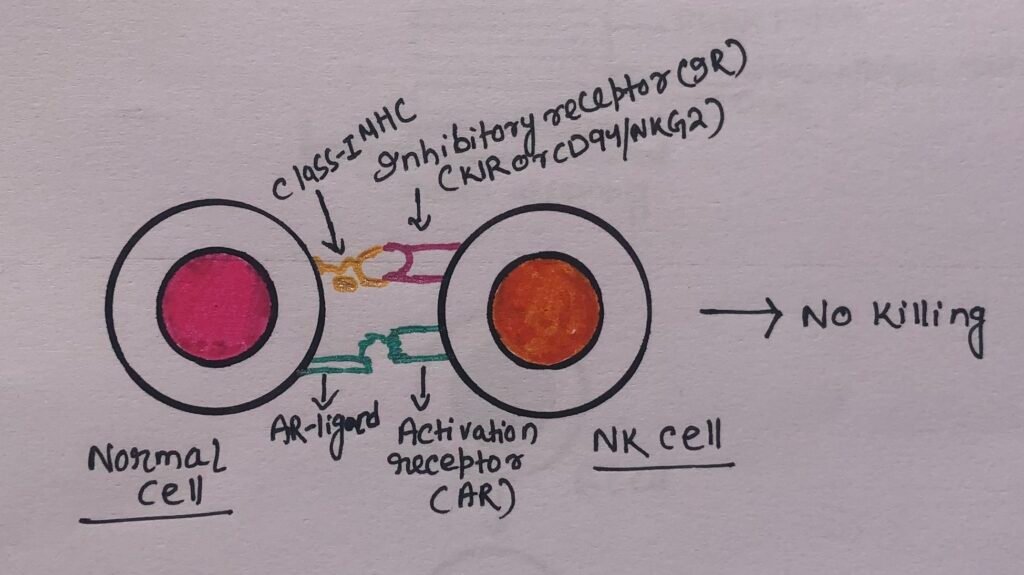
The expression of class I MHC molecules on normal cells prevents their destruction by NK cells. However, the decreased class I MHC expression on altered-self cells promotes the cell-killing activity of NK cells.
Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC)- A mechanism of cell-mediated immune response
It is a mechanism of cell-mediated immune defense, where an effector cell kills a target cell that has been bound by specific antibodies. ADCC requires an effector cell, specifically NK cells, which typically interact with IgG antibodies. Macrophages, eosinophils, and neutrophils can also mediate antibody-dependent cell-mediated cytotoxicity. Many cells having cytotoxic potential, express membrane receptors for the Fc region of the antibody molecule. When the antibody is specifically bound to the surface of a pathogen-infected target cell, then the receptor-bearing cells can bind to the antibody Fc region and cause the lysis of the target cell.
ADCC by NK cell
NK cells express a membrane molecule CD16, which has a Fc receptor for the antibody IgG. The Fc receptor of CD16 binds to its compatible region of the antibody IgG. After the attachment, the natural killer cell releases cytokines such as IFN-γ, tumor necrosis factor (TNF), and cytotoxic granules containing perforin and granzymes. The granules enter the target cell and promote cell death by apoptosis (Figure 4). Macrophages, neutrophils, or eosinophils bind to a target cell through the Fc receptor. After binding, they become more metabolically active. Thus, the lytic enzymes at the site of the Fc-mediated contact may result in damage to the target cell.
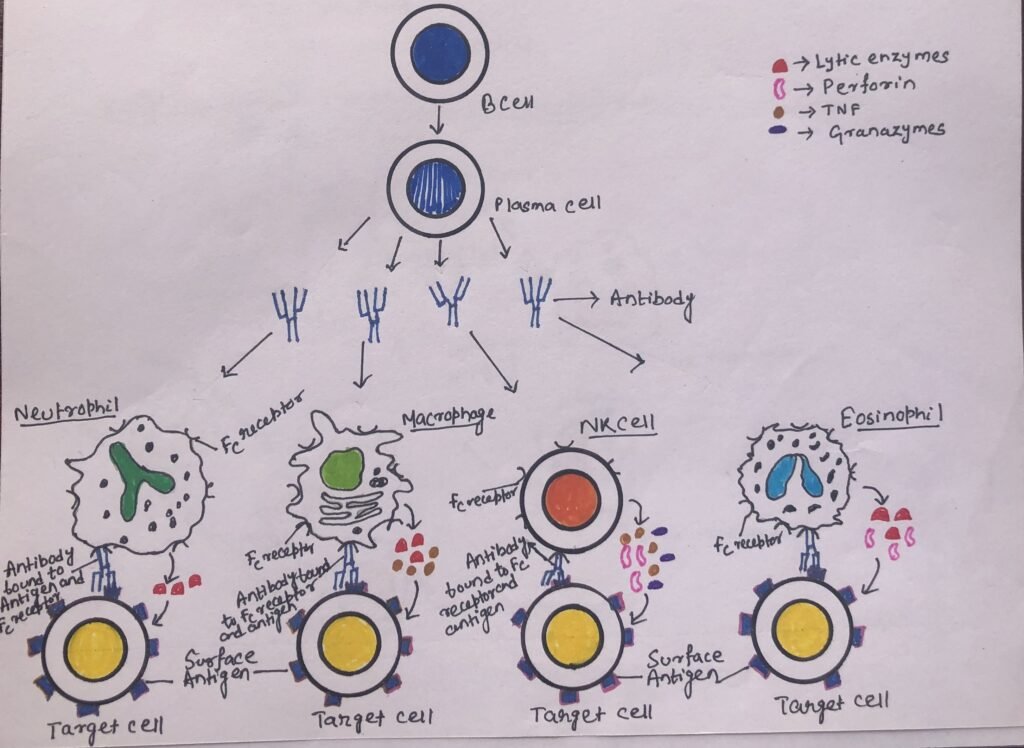
ADCC by eosinophils
Through antibody-dependent cell-mediated cytotoxicity, eosinophils can kill certain parasitic worms and helminths. Phagocytes can not engulf helminths due to their large size. The helminths are covered by an external structure, integument, which provides resistance to substances released by neutrophils and macrophages. The Fc receptor of an eosinophil can recognize the antibody IgE, which coats these parasites. The degranulation of eosinophils (Figure 4) is caused by the interaction between the Fc receptor and the Fc portion of the helminth-bound antibody IgE.
Delayed-type hypersensitivity (DTH)
The delayed-type hypersensitivity is an effector response mediated by inflammatory cells. However, the response is controlled specifically by the TH1 subset of T helper cells. When some subpopulations of activated T cells encounter certain types of antigens, they secrete cytokines. The secreted cytokines induce a localized inflammatory reaction called delayed-type hypersensitivity. The DTH response involves the macrophages as the principal effector cells.
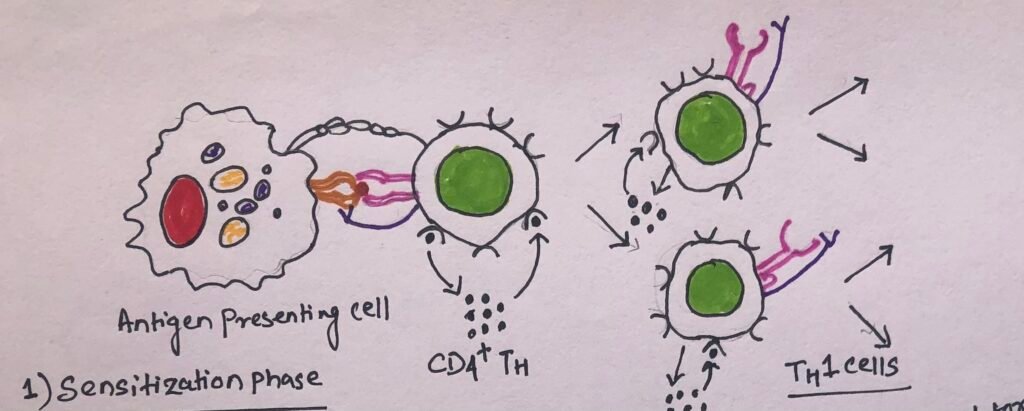
The initial sensitization phase
The development of the DTH response begins with an initial sensitization phase. The sensitization phase is of duration 1-2 weeks after primary contact with an antigen (Figure 5). This phase involves the activation and clonal expansion of the T helper cells by antigen presented together with the requisite class II MHC molecule on an appropriate antigen-presenting cell.
The effector phase
The second phase of the DTH response also known as the effector phase, begins with a subsequent exposure to the antigen. In this phase, TH1 cells secrete a variety of cytokines that recruit and activate macrophages (Figure 6) and other non-specific inflammatory cells.
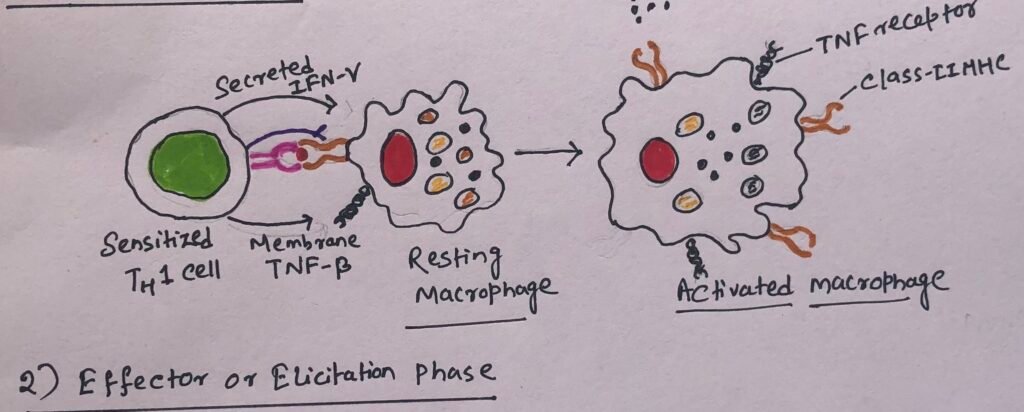
Cytokines secreted by TH1 cells induce blood monocytes to migrate from the blood into the surrounding tissues. The monocytes get differentiated into activated macrophages. Activated macrophages function as effective antigen-presenting cells by expressing increased levels of class II MHC molecules and cell-adhesion molecules. In the site of a DTH reaction, macrophages begin to accumulate and are activated by cytokines. The cytokines mainly include IFN-γ and membrane-bound TNF-β produced by TH1 cells. The activated macrophages activate more T cells, which in turn secrete more cytokines that recruit and activate even more macrophages.
Secretion of lytic enzymes and the phagocytic activity of macrophages in the infection area lead to non-specific destruction of cells and, thus of the intracellular pathogen. However, when the antigen is not easily cleared, a prolonged DTH response can itself become destructive to the host. This is because of the development of the intense inflammatory response into a visible granulomatous reaction. Thus, this response leads to tissue necrosis.
Conclusion
Effector cells are the cells of the adaptive immune system, carrying out an immune function in response to a stimulus. The first group includes the antigen-specific cytotoxic T lymphocytes (CTLs). The second group includes the nonspecific cells, i.e., the NK cells and macrophages.
The mechanism of cell killing by a CTL begins when it first makes direct contact with its target cell through the T cell receptor (TCR) and class I MHC molecules, respectively. CTLs can kill the target cell either by the perforin/granzyme pathway or by the Fas pathway.
The natural killer (NK) cells can efficiently kill target cells in an antibody-independent manner. The natural killer cells are cytotoxic as they possess granules containing perforin and granzyme in their cytoplasm. When they interact with a target cell, degranulation with the release of perforin and granzymes takes place at the junction of interaction.
ADCC is a mechanism where an effector cell kills a target cell that has been bound by specific antibodies. NK cells, macrophages, eosinophils, and neutrophils can mediate antibody-dependent cell-mediated cytotoxicity. When the antibody is specifically bound to the surface of a pathogen-infected target cell, then the receptor-bearing cells can bind to the antibody Fc region. This causes the lysis of the target cell.
The delayed-type hypersensitivity is an effector response mediated by inflammatory cells. The response involves two phases, i.e., the initial sensitization phase and the effector phase. The sensitization phase involves the activation and clonal expansion of the T helper cells by antigen presented together with the requisite class II MHC molecule on an appropriate antigen-presenting cell. The effector phase begins with the subsequent exposure to the same antigen.
You may also like:
- The delayed-type hypersensitivity: a cell-mediated response
- Contraction of our immune response and the response of effector and memory lymphocytes

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around twelve years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.
