In this article, I briefly describe some proteins that facilitate the transbilayer movement of a lipid molecule.
The Lipid Bilayer
The lipid bilayer is the core structure of all biological membranes, creating a flexible boundary that encloses cells and their internal compartments. It is mainly made of phospholipids, which naturally organize into a double layer in watery environments. This is possible because of their amphipathic properties. This bilayer is dynamic and self-organizing. It maintains cell structure, enables communication, and supports various cellular functions. Its distinct amphipathic nature allows it to act as a flexible and selectively permeable barrier, essential for sustaining life.
The structure of the lipid bilayer is stable, but the individual phospholipid molecules within it can move quite freely, depending on factors like temperature and lipid makeup. At temperatures below the normal physiological range, the lipids adopt a gel-like liquid-ordered (L₀) state (Figure 1). In this state, the movement of individual molecules is highly restricted, and the bilayer exhibits a paracrystalline organization.
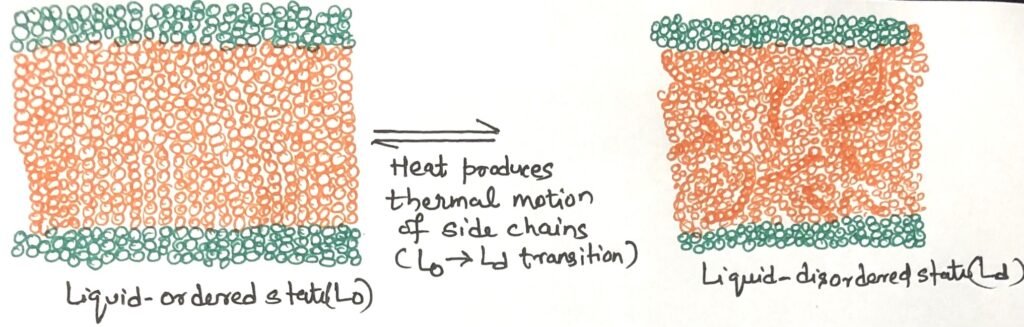
At temperatures above the physiological range, the hydrocarbon chains of fatty acids exhibit continuous movement, driven by rotation around the carbon-carbon bonds of their extended acyl chains and by lateral diffusion of lipid molecules within the plane of the bilayer. This dynamic condition is the liquid-disordered (Ld) state (Figure 1). During the shift from the L₀ state to the Ld state, the overall structure and size of the bilayer remain unchanged.
Catalysis Helps the Transbilayer Movement of Lipids
At physiological temperatures, the movement of a lipid molecule from one leaflet of the bilayer to the opposite occurs very slowly (Figure 2). However, in most membranes, lateral diffusion within the plane of the bilayer happens quite rapidly (Figure 3). The transbilayer movement, often called flip-flop, involves the polar or charged head group moving out of its aqueous surroundings into the hydrophobic core of the bilayer—an energetically unfavorable process with a significant positive free-energy change.
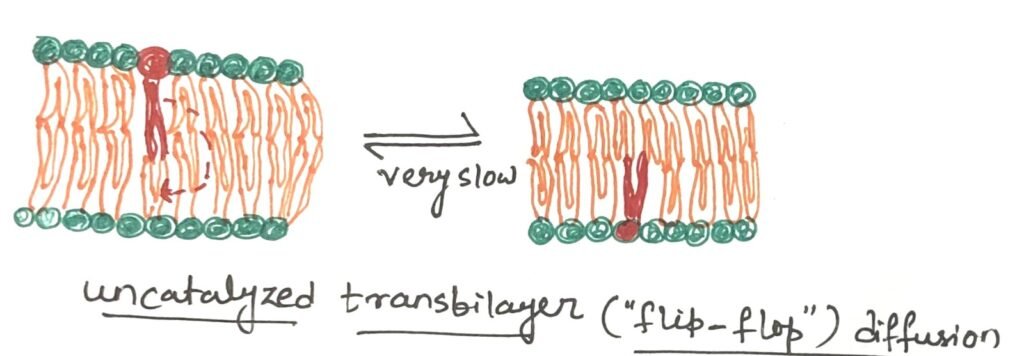
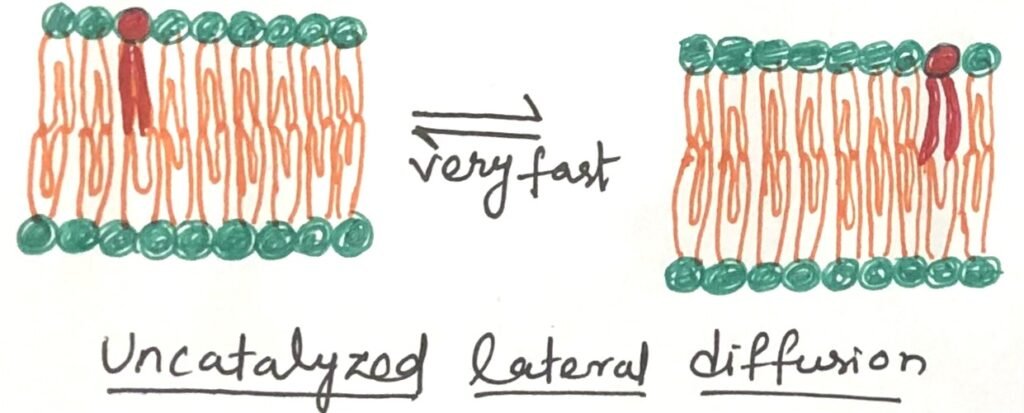
Proteins Aid in the Transbilayer Movement of a Lipid Molecule
Flippases, floppases, and scramblases are the specialized proteins that facilitate the transbilayer movement of individual lipid molecules (Figure 3). They provide a more energetically favorable and much faster route when compared to the slow, uncatalyzed transbilayer movement. The combination of asymmetrical lipid synthesis, the inherently slow natural flip-flop diffusion, and the action of selective, energy-dependent lipid translocators helps maintain the lipid asymmetry across the bilayer. In addition to establishing this asymmetry, the energy-driven transport of lipids to one leaflet may also expand the surface area on one side of the membrane, playing a crucial role in generating membrane curvature needed for vesicle formation.
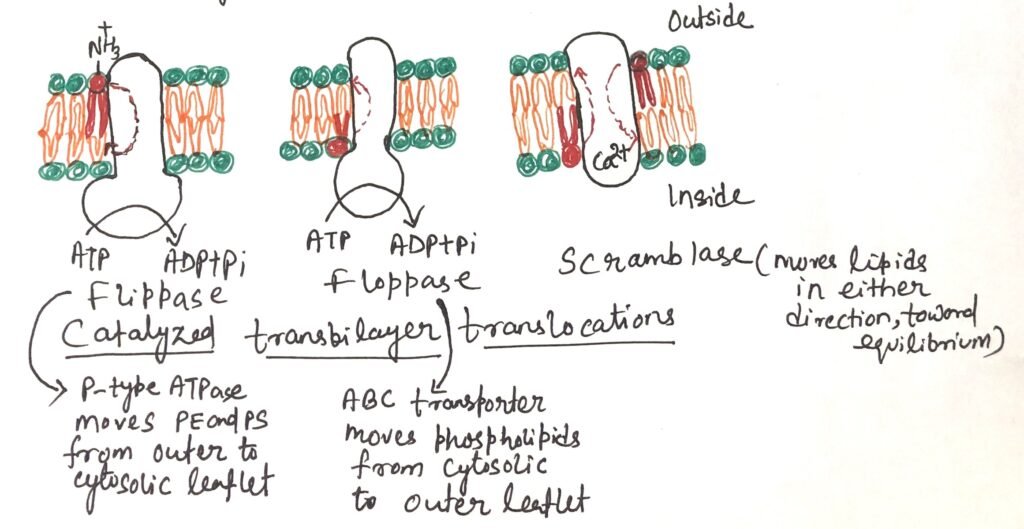
Working of Flippases
Flippases are proteins that catalyze the movement of aminophospholipids—specifically phosphatidylethanolamine (PE) and phosphatidylserine (PS) —from the outer (extracellular) leaflet to the inner (cytosolic) leaflet of the plasma membrane. This activity helps maintain the asymmetric distribution of phospholipids, with phosphatidylserine and phosphatidylcholine concentrated in the cytosolic leaflet, while sphingolipids and phosphatidylcholine are predominantly found in the outer leaflet. The exposure of phosphatidylserine on the outer surface triggers apoptosis. Macrophages with phosphatidylserine receptors carry out engulfment.
Flippases also function within the endoplasmic reticulum, where they help move new phospholipids from the side of the membrane facing the cytosol to the side facing the lumen of the ER. This process uses about one ATP molecule for each phospholipid moved. Flippases are similar in structure and function to P-type ATPases.
Working of Floppases
Plasma membrane phospholipids and sterols move from the cytosolic to the extracellular leaflet with the help of floppases. Similar to flippases, floppases require ATP to function. They belong to the ABC transporter family, whose members actively transport hydrophobic molecules outward across the membrane. Floppases play an important role in maintaining membrane lipid asymmetry and are involved in various cellular processes such as membrane trafficking, cell signaling, and the formation of lipid rafts. Additionally, by regulating the distribution of lipids, floppases contribute to the proper functioning of membrane proteins and influence interactions between the cells.
Working of Scramblases
Scramblases are proteins that move membrane phospholipids across the bilayer following their concentration gradient. Unlike flippases and floppases, scramblases do not require ATP. Their function causes a controlled mixing of phospholipid head groups between the two sides of the membrane. During cell activation, injury, or apoptosis, scramblase activity increases significantly. The cytosolic Ca²⁺ levels rise during these processes, causing the rise in scramblase activity. An example is rhodopsin, a light-sensitive protein in the vertebrate eye, which also acts as a scramblase capable of rapidly redistributing over 10,000 phospholipids per protein per second.
Conclusion
The lipid bilayer, composed of amphipathic phospholipids, forms the core of biological membranes. It creates a dynamic, flexible, and selectively permeable barrier that maintains cell structure and supports vital cellular functions. The lipid bilayer is stable yet dynamic, with phospholipids moving freely depending on temperature and composition. Below physiological temperatures, it forms a gel-like liquid-ordered (L₀) state with restricted movement. Above this range, it shifts to a liquid-disordered (Ld) state. In this state, fatty acid chains rotate and lipids diffuse laterally, while the bilayer’s overall structure remains intact.
Flippases, floppases, and scramblases are the specialized proteins that facilitate the transbilayer movement of individual lipid molecules. They provide a more energetically favorable and much faster route when compared to the slow, uncatalyzed transbilayer movement.
Flippases are proteins that catalyze the movement of aminophospholipids—specifically phosphatidylethanolamine and phosphatidylserine—from the outer (extracellular) leaflet to the inner (cytosolic) leaflet of the plasma membrane.
Floppases assist in transferring phospholipids and sterols from the cytosolic to the extracellular leaflet of the plasma membrane. Like flippases, their activity is ATP-dependent. Floppases are part of the ABC transporter family, which actively moves hydrophobic molecules outward through the membrane.
Scramblases are proteins that facilitate the movement of phospholipids across the membrane bilayer along their concentration gradient. They do not require ATP. Their action leads to a regulated redistribution of phospholipid head groups between both sides of the membrane. Their activity rises sharply during cell activation, damage, or apoptosis.
You may also like:
- Peptides and proteins with their distinguishing properties
- Triacylglycerols: Energy storage, insulation, and health impacts of partial hydrogenation

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.




