In this article, I briefly describe phagocytes- the white blood cells in our immune system. They play a critical role in defending the body against infections.
Phagocytes
Phagocytes were first identified in 1882 by Ilya Ilyich Mechnikov during his research on starfish larvae. These white blood cells protect the body by engulfing and breaking down harmful particles, bacteria, and dead or damaged cells. Phagocytes are essential for infection control and immunity, and they are found widely throughout the animal kingdom, especially well-developed in vertebrates. In humans, a single liter of blood contains around six billion phagocytes. Phagocytes are generally categorized based on whether they have surface receptors. Professional phagocytes with receptors can recognize and target harmful organisms effectively. Professional phagocytes are differentiated into neutrophils, monocytes, macrophages, dendritic cells, and mast cells.
Neutrophils
Neutrophils are the granulocytes produced by hematopoiesis in the bone marrow. They are the most abundant type of phagocyte normally found in the bloodstream, constituting 50% to 60% of the total circulating white blood cells. One liter of human blood contains about five billion neutrophils, which are about 10 micrometers in diameter and live for only about five days. The neutrophil has a multilobed nucleus (figure 1) and a granulated cytoplasm that stains with both acidic and basic dyes. Neutrophils are the first to reach an inflammation site. Bone marrow releases more neutrophils at the site of an infection.
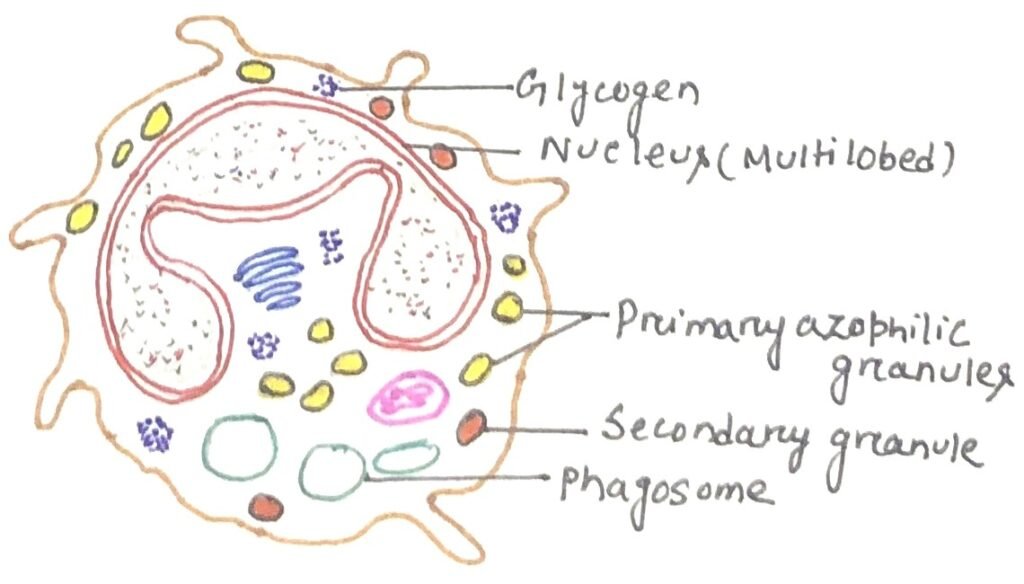
Extravasion is the process where circulating neutrophils move into tissues. In this process, the cell first adheres to the vascular endothelium. Then, it penetrates the gap between adjacent endothelial cells. Finally, it pierces the vascular basement membrane, moving out into the tissue spaces. As ferocious eaters neutrophils rapidly engulf invaders coated with antibodies and complement, and damaged cells or cellular debris. Neutrophils do not come back to the blood as they turn into pus cells and die.
These also employ both oxygen-dependent and oxygen-independent pathways to generate antimicrobial substances. Neutrophils exhibit a larger respiratory burst than macrophages and consequently are able to generate more reactive oxygen intermediates and reactive nitrogen intermediates.
Monocytes
Monocytes are a type of white blood cell and an integral part of the innate immune system in vertebrates, including all mammals (humans included), as well as birds, reptiles, and fish. During hematopoiesis in the bone marrow, granulocyte-monocyte progenitor cells develop into promonocytes, which then exit the bone marrow and enter the bloodstream, where they further mature into monocytes.
Monocytes are the largest cells among blood corpuscles and are typically recognized in stained smears by their large, kidney-shaped, or notched nucleus. They circulate in the bloodstream for around eight hours, during which they grow larger and then typically migrate into various body tissues. Monocytes make up about three to eight percent of leukocytes in the blood, with half of them stored as a reserve in clusters within the red pulp’s Cords of Billroth in the spleen. Once in tissues, monocytes develop into different types of macrophages depending on their location in the body.
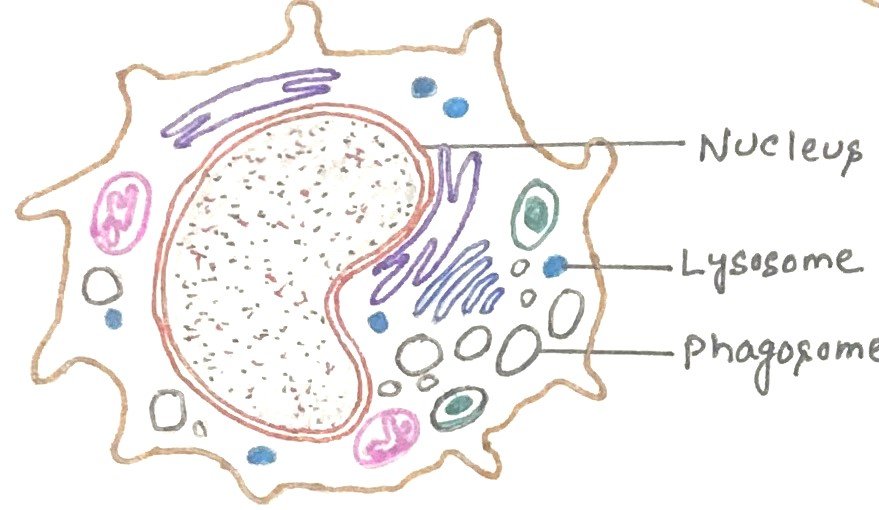
Differentiation of a monocyte into tissue macrophage
When a monocyte is ready to differentiate, it undergoes several changes. The cell enlarges five to tenfold as its intracellular organelles increase in both number and complexity. It acquires increased phagocytic ability and produces higher levels of hydrolytic enzymes and soluble factors.
Monocytes are drawn to sites of tissue damage through chemotaxis, a process initiated by various signals, including damaged cells, pathogens, and cytokines released by macrophages already present at the site. In the immune system, monocytes play key roles, such as phagocytosis, antigen presentation, and cytokine production.
Phagocytosis involves engulfing microbes and particles, followed by their digestion and destruction. Monocytes can perform phagocytosis using opsonizing proteins like antibodies or complement that coat pathogens, or by directly binding to microbes via pattern-recognition receptors that identify pathogens. They are also capable of destroying infected host cells through antibody-mediated cellular cytotoxicity.
Typical cytokines produced by monocytes include tumor necrosis factor (TNF), interleukin-1 (IL-1), and interleukin-12 (IL-12). Human blood contains three monocyte subtypes: classical monocytes, which express high levels of the CD14 cell surface receptor; non-classical monocytes, which have low CD14 expression and coexpress the CD16 receptor; and intermediate monocytes, characterized by high CD14 and low CD16 expression.
Macrophages
Monocytes differentiate in tissues to macrophages. Tissue macrophages reside in particular tissues while free macrophages are motile. Human macrophages are about 21 micrometers (0.00083 in) in diameter. Macrophages function in both innate and adaptive immunity of vertebrate animals.
Macrophages serve different functions in different tissues and are named according to their tissue location. They are called alveolar macrophages in the lungs, histiocytes in connective tissues, Kupffer cells in the liver, mesangial cells in the kidney, microglial cells in the brain, and osteoclasts in the bone. These phagocytose (engulf and then digest) cellular debris and pathogens, either as stationary or as mobile cells. They stimulate lymphocytes and other immune cells to respond to pathogens. Macrophages are specialized phagocytic cells that attack foreign substances, infectious microbes, and cancer cells through destruction and ingestion. They move by the action of amoeboid movement (figure 3).
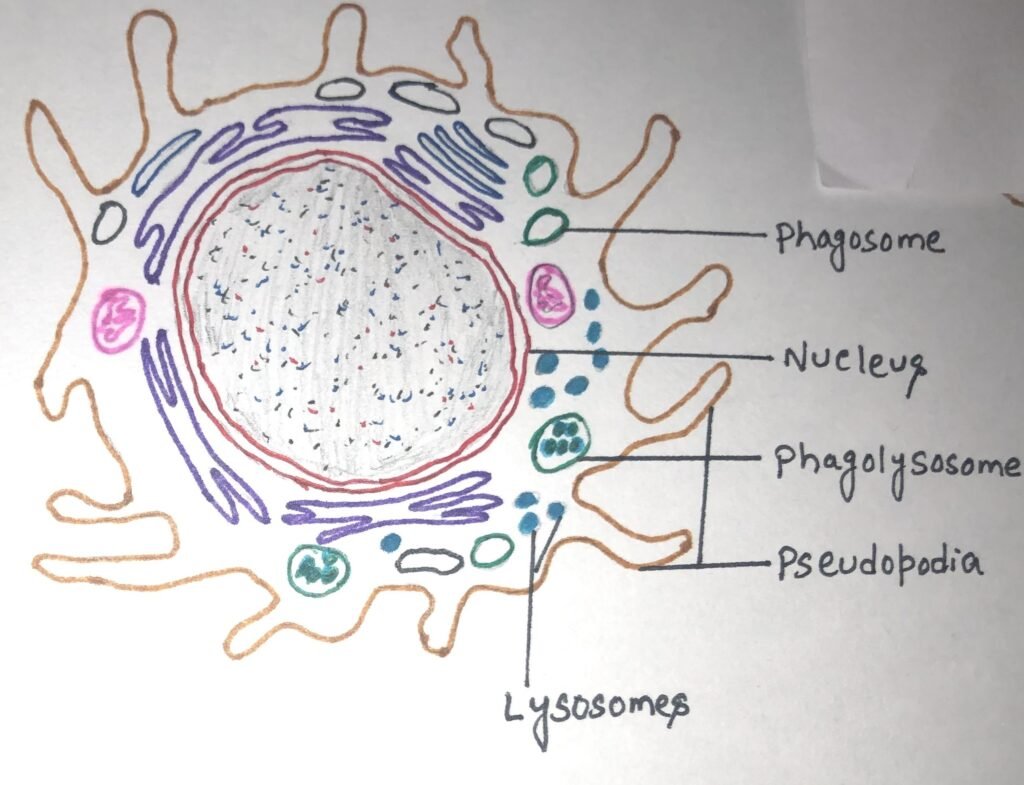
When macrophages phagocytose particulate antigens, it acts as an initial activating stimulus. However, macrophages can be activated by various stimuli in the course of an immune response. Macrophages can be activated to perform functions that a resting monocyte cannot. TH1 cells activate macrophages by signaling with IFN-gamma and displaying the protein CD40 ligand. Activated macrophages exhibit more phagocytic activity, thus more effectively eliminating potential pathogens than the resting pathogens. Various cytotoxic proteins secreted by activated macrophages help eliminate a broad range of pathogens including virus-infected cells, tumor cells, and intracellular bacteria.
Macrophages carry out phagocytosis
During phagocytosis, macrophages are attracted to and migrate toward a range of substances produced as part of the immune response, a process called chemotaxis. Antigens then adhere to the macrophage’s cell membrane, guided by membrane protrusions on the macrophage, known as pseudopodia ( figure 3). Pseudopodia extend around the bound material, and their fusion encloses it within a membrane-bound compartment called a phagosome, which proceeds along the endocytic pathway. The phagosome then merges with a lysosome to form a phagolysosome, where lysosomal enzymes, such as lysozyme and other hydrolytic enzymes, digest the ingested material.
Respiratory burst in activated macrophages
During phagocytosis, a metabolic event called the respiratory burst occurs in activated macrophages, leading to the activation of a membrane-bound oxidase that reduces oxygen to superoxide anion, a reactive oxygen intermediate highly toxic to engulfed microorganisms. This superoxide anion also produces strong oxidizing agents, such as hydroxyl radicals and hydrogen peroxide. When macrophages are stimulated by bacterial cell wall components like lipopolysaccharide or muramyl dipeptide along with a T-cell cytokine (IFN-γ), they express high levels of nitric oxide synthase, an enzyme that converts L-arginine into L-citrulline and nitric oxide. Nitric oxide has powerful antimicrobial effects and, when combined with superoxide anion, forms even more potent antimicrobial agents.
Additionally, activated macrophages produce lysozymes and various hydrolytic enzymes whose degradative actions do not depend on oxygen. They also synthesize antimicrobial and cytotoxic peptides known as defensins, which create ion-permeable channels in bacterial cell membranes. Macrophages as scavengers, rid the body of worn-out cells and other debris. They are the leading antigen-presenting cells along with dendritic cells. Monocytes and macrophages are secretory cells and are vital to the regulation of immune responses and the development of inflammation. They produce a wide array of powerful chemical substances (monokines) including enzymes, complement proteins, and regulatory factors such as interleukin-1.
Eosinophils
Eosinophils are granulocytes that develop during hematopoiesis in the bone marrow. They are white blood cells (figure 4) responsible for combating multicellular parasites and certain infections in vertebrates. Eosinophils, like neutrophils, are motile phagocytic cells that can migrate from the blood into the tissue spaces.
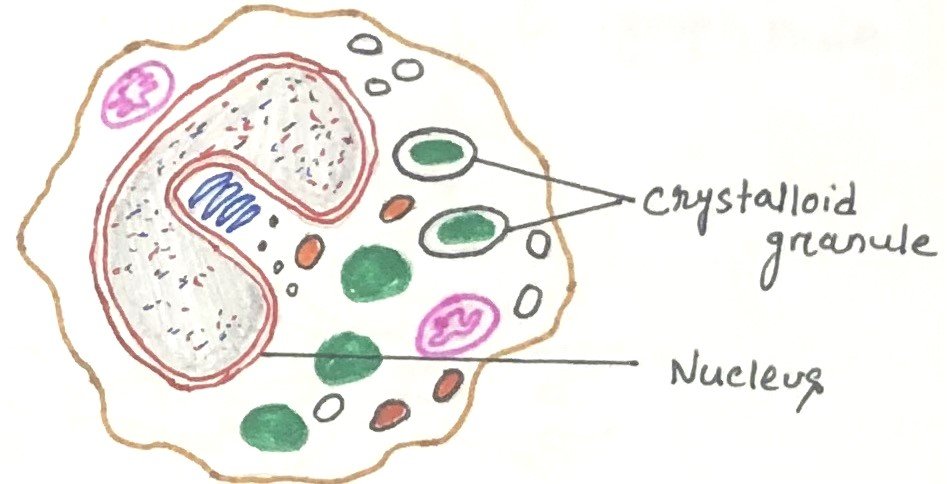
In normal individuals, eosinophils make up about 1-6% of white blood cells and are about 12-17 micrometers in size.
Eosinophils appear brick-red when stained with eosin, a red dye, through the Romanowsky method. The staining highlights numerous small granules in the cytoplasm, which contain various chemical mediators, including histamines and proteins like eosinophil peroxidase, ribonuclease (RNase), deoxyribonucleases, lipase, plasminogen, and major basic protein. Upon activation, eosinophils release these mediators via degranulation, which is harmful to both parasites and host tissues. They are present in the thymus’s medulla and the junction between the cortex and medulla, as well as in the lower gastrointestinal tract, ovaries, uterus, spleen, and lymph nodes. In circulation, eosinophils last for 8–12 hours, but they can survive in tissue for another 8–12 days if unstimulated.
Eosinophils contain an abundance of RNases within their granules, which help them fight viral infections and in fibrin removal during inflammation. Eosinophils along with basophils and mast cells, are important mediators of allergic responses They also fight helminth (worm) colonization and may be slightly elevated in the presence of certain parasites.
Mast cells
The precursors of mast cells are formed in the bone marrow by hematopoiesis. They are released into the blood as undifferentiated cells and when they enter into the tissues, they start differentiating. A mast cell contains many granules rich in histamine and heparin. Mast cells are found in a wide variety of tissues including the skin, connective tissues of various organs, and mucosal epithelial tissue of the respiratory, genitourinary, and digestive tracts.
Mast cells play a key role in the inflammatory process. These cells can be stimulated to degranulate by direct injury (e.g. physical or chemical [such as opioids, alcohols, and certain antibiotics such as polymyxins]), cross-linking of Immunoglobulin E (IgE) receptors, or by activated complement proteins.
They express a high-affinity receptor (FceRI) for the Fc region of IgE antibody. In allergic reactions, mast cells remain inactive until an allergen binds to IgE already in association with the cell. Allergens are nonparasitic antigens capable of stimulating type I hypersensitivity responses in allergic individuals. When an individual gets exposed to a parasite, serum IgE levels increase and remain high until the parasite is completely cleared from the body. However, some persons may have an abnormality called atopy, a hereditary predisposition to the development of immediate hypersensitivity reactions against common environmental antigens. Atopic individuals have abnormally high levels of circulating IgE, thus they are more susceptible to allergies.
Mast cells play a role in type I hypersensitive reaction
Allergens induce a type I hypersensitivity reaction (figure 5), where an allergen induces a humoral antibody response, resulting in the generation of antibody-secreting plasma cells and memory cells. Plasma cells secrete IgE. This class of antibody binds with high affinity to Fc receptors on the surface of tissue mast cells and blood basophils. After binding of IgE antibodies, the mast cells and basophils remain sensitized (figure 5). When an individual again gets exposed to the same allergen, it cross-links the membrane-bound IgE on sensitized mast cells and basophils, causing degranulation of these cells. The pharmacologically active mediators released from the granules act on the surrounding tissues. These chemical mediators cause the characteristic symptoms of allergy.

Mast cells can consume and kill gram-negative bacteria (e.g., salmonella), and process their antigens. Additionally, mast cells produce cytokines that induce an inflammatory response. The cytokines attract more phagocytes to the infection site and microbes are destroyed.
Dendritic cells
These cells play a vital role as antigen-presenting cells. Dendritic cells are covered with long membrane extensions that resemble the dendrites of nerve cells. They act as messengers between the innate and adaptive immunity. Dendritic cells are derived from hematopoietic bone marrow progenitor cells. These progenitor cells initially transform into immature dendritic cells. These cells have high endocytic activity and low T-cell activation potential. Immature dendritic cells constantly sample the surrounding environment for viruses and bacteria, which is done through pattern recognition receptors (PRRs) such as the toll-like receptors (TLRs).
These immature dendritic cells when come in contact with a presentable antigen, they become activated into mature dendritic cells and begin to migrate to the lymph node. Immature dendritic cells phagocytose pathogens and degrade their proteins into small pieces. When they mature, present those fragments at their cell surface using an MHC molecule. Mature dendritic cells activate T helper cells and cytotoxic T cells. The activated helper T cells interact with macrophages and B cells to activate them in turn. The four types of dendritic cells can be categorized as Langerhans cells, interstitial cells, myeloid cells, and lymphoid dendritic cells. Each arises from hematopoietic stem cells via different pathways and in different locations. All types of dendritic cells express high levels of both class II MHC molecules and members of the co-stimulatory B7 family.
A type of dendritic cell called the follicular dendritic cell doesn’t arise in bone marrow and has a different function from the antigen-presenting dendritic cells. Follicular dendritic cells can’t act as antigen-presenting cells as they don’t express class-II MHC molecules. These cells reside in lymph follicles, rich in B cells. Follicular dendritic cells express high levels of membrane receptors for antibodies, which allow the binding of antigen-antibody complexes.
Conclusion
Phagocytes are white blood cells, that play a crucial role in protecting the body by engulfing and breaking down harmful particles, bacteria, and dead or damaged cells. They are essential for infection control and immunity and are found widely throughout the animal kingdom, being especially well-developed in vertebrates. Professional phagocytes are differentiated into neutrophils, monocytes, macrophages, dendritic cells, and mast cells.
Neutrophils are the granulocytes produced by hematopoiesis in the bone marrow. They are the most abundant type of phagocyte normally found in the bloodstream. They constitute 50% to 60% of the total circulating white blood cells. Monocytes are a type of white blood cell and an integral part of the innate immune system in vertebrates. Monocytes differentiate in tissues to macrophages. During phagocytosis, macrophages are attracted to and migrate toward a range of substances produced as part of the immune response, a process called chemotaxis.
Eosinophils, like neutrophils, are motile phagocytic cells that can migrate from the blood into the tissue spaces. A mast cell contains many granules rich in histamine and heparin. Mast cells play a key role in the inflammatory process. These cells can be stimulated to degranulate by direct injury, cross-linking of Immunoglobulin E (IgE) receptors, or by activated complement proteins.
Dendritic cells play a vital role as antigen-presenting cells. They act as messengers between the innate and adaptive immunity. The four types of dendritic cells can be categorized as Langerhans cells, interstitial cells, myeloid cells, and lymphoid dendritic cells.
You may also like:

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.



