In this article, I briefly describe the types of receptors in our immune system.
Receptors of the immune system
The cells of our immune system use various receptors to recognize ligands. Some receptors, like pattern recognition receptors (PRRS), are found in both innate and adaptive immune cells. However, B-cell receptors (BCRs) and T-cell receptors (TCRs) are antigen-specific receptors found only in the adaptive immune system on the surface of B and T lymphocytes, respectively.
The number of innate immune receptors varies around a hundred or less in number, whereas adaptive immune receptors are present in ample amounts around units of billions. Adaptive receptors express diverse antigen binding sites, which are enabled by the unique ways T cell and B cell receptors are encoded in the genome.
The locations of antigen receptors in the innate immune system are markedly more variable than adaptive antigen receptors. Some innate immune receptors are completely soluble, e.g., mannose-binding lectin (MBL) circulating in tissue fluids.
However, members of the TLR family (innate receptors) may associate with the cytosol-facing members of intracellular endosomes and lysosomes, which are specific for molecules of cytosol or may attach to the plasma membrane and bind antigens as the lipopolysaccharides of gram-ve bacteria.
B cell receptor exists in both membrane-bound and soluble form
Among all the receptors of the innate and adaptive immune system, the B-cell receptor is different from others as it can make both soluble and membrane-bound forms of an immunoglobulin receptor sharing the same antigen binding site and are encoded by the same gene.
An antigen stimulates a B cell to secrete an antibody (the soluble form of a BCR). The B-cell receptor entails a membrane-bound immunoglobulin molecule along with a signal transduction moiety. The activation of a B cell is controlled by the B-cell receptor.
The structure of antibody
Both soluble antibodies and membrane-bound B-cell receptors are included in the immunoglobulin family of proteins. These consist of two identical heavy and two identical light chains. In the membrane-bound receptor, the receptor is anchored into the plasma membrane by the hydrophobic residues of the carboxyl ( C ) terminus of the heavy chain.
The daughter cells of the original B cell start to secret soluble antibodies after being stimulated by an antigen. In this process, at the C-terminal end of the antibody, hydrophobic residues are exchanged for more hydrophilic amino acid residues.
The carboxyl terminus of the heavy chains holds the biochemical difference between the membrane-bound receptor and the secreted form of the antibody.
Secreted antibodies possess a hydrophilic amino acid sequence of variable lengths at the carboxyl terminus. However, this sequence is replaced by three sequentially arranged regions in a membrane-bound immunoglobulin receptor.
The three sequences include an extracellular hydrophilic ”spacer” sequence of approximately 26 amino acids, a hydrophobic transmembrane segment of approximately 25 amino acids, and a too-short cytoplasmic tail.
One B cell can make two different varieties of mRNA encoding the immunoglobulin heavy chain. The coding information for the hydrophilic C-terminus of the secreted antibody is expressed by one form of m-RNA. The other form of mRNA expresses the information for the hydrophobic C-terminus of the membrane-bound receptor.
A resting B-cell enables the mRNA to code only the membrane-bound form. When it gets activated by an antigen, the RNA splicing machinery receives signals from the antigen receptor to make both types of heavy-chain mRNA.
Comparison between the structure of antibody and BCR
The B-cell receptors share the same structure with the antibodies. The structure entails four polypeptide chains consisting of two identical light (L) chains and two identical heavy (H) chains. Each heavy chain and light chain are connected by a disulfide bond.
The two heavy chains are connected through disulfide bonds located outside antigen-binding regions. The antibody molecule has two antigen binding sites, made up of components of both heavy and light chains.
BCR co-receptors take part in the antigen-binding activity
The co-receptors modulate the activation of signaling molecules, which essentially transmit a signal through the antigen receptor. It helps in ligand recognition and initiation of biological processes.
On mature B cells, the B-cell coreceptor is expressed as a complex of three transmembrane molecules, CD19, CD21, and CD81. CD21 acts as a co-receptor as it takes part in the antigen-binding activity of the BCR complex. CD19 and CD81 molecules, instead of binding to antigens, rather participate in the passage of an antigen signal across the B-cell membrane.
Our innate immune system first recognizes an antigen as foreign before its binding with BCR and CD21 coreceptor. The innate immune system helps to covalently bind a C3d (complement protein) protein fragment to the target antigen.
Then, the CD21 co-receptor molecule binds to C3d, and the target antigen binds directly to BCR and indirectly through the binding of CD21 and C3d. This increases the avidity of antigen-binding to the cell (Figure 1).

T-cell receptors
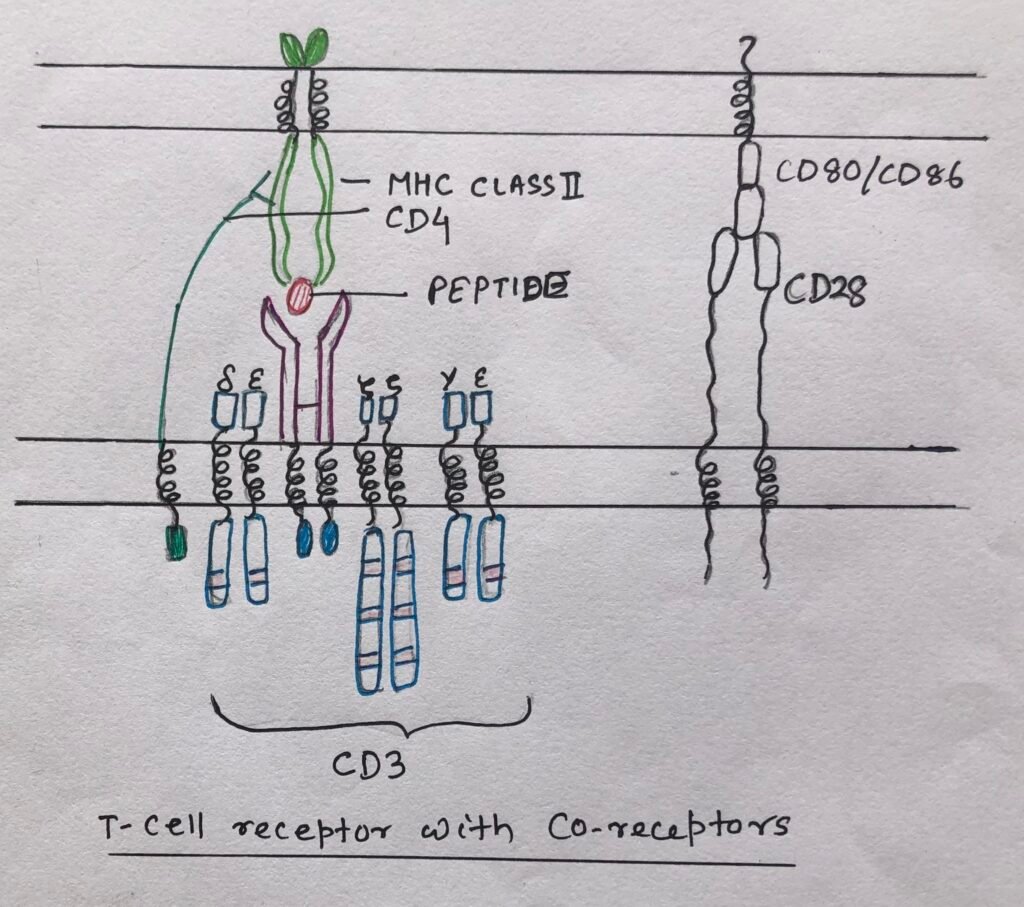
T-cell receptors are membrane-bound receptors. Unlike B-cell receptors, they are not secreted in soluble form. TCRs bind antigens, which are presented by antigen-presenting cells in the form of a complex.
The antigens, in complex with plasma-membrane bound major-histocompatibility complex, bind to most T-cell receptors. The T-cells, recognizing MHC-presented peptides, bear a heterodimeric receptor made up of an α chain and a β chain (αβ TCR). The T-cells, bearing heterodimeric γδ receptor, recognize antigens, which may not be presented by MHC-proteins.
TCR chains with two immunoglobulin domains
The TCR chain consists of two immunoglobulin domains. Among the two, one is the variable domain at the N-terminus, which serves as the antigen-binding site. The other one is a constant domain, which puts the antigen-binding site away from the plasma membrane.
Each variable domain has three complementary determining regions having contact with the antigenic complex. In X-ray crystallographic analysis, TCR antigen complexes show an association of MHC proteins with CDR1 and CDR2 regions of the αβ TCR. Whereas CDR3 regions mainly interact with the antigenic peptide.
Heterodimer Igα, Igβ mediating signal transduction
A BCR heavy chain consists of an extremely short cytoplasmic tail but still passes signal into the cytoplasm. This happens because of the non-covalent association of BCR with a heterodimer Igα, Igβ. This non-covalent association transduces the antigen signal into the cytoplasm.
The BCR complex has two components, a recognition component, including BCR and CD21, and a signal transduction component, which includes Igα and Igβ.
Igα and Igβ are transmembrane proteins having short, N-terminal regions and long intracytoplasmic tails containing ITAMs. ITAMs are immunoreceptor tyrosine-based activation motifs containing short sequences of amino acids that include two tyrosine residues.
When the associated BCR molecule binds to a ligand, it gets activated, leading to the phosphorylation of the tyrosine residues. Then, the residues bind with the downstream signaling molecules.
Functioning of TCR co-receptors
The αβ TCR (Figure 2) like BCR, associates non-covalently with several co-receptors on the cell surface. The CD4 co-receptor, a monomeric membrane glycoprotein, contains four extracellular Ig-like domains, a hydrophobic transmembrane region, and a long cytoplasmic tail.
The CD8 co-receptor is a disulfide-linked αβ heterodimer or αα homodimeric glycoprotein. Each chain has one extracellular immunoglobulin-like domain, a transmembrane part, a stalk region, and a cytoplasmic tail.

Sometimes, a naïve T cell is not getting activated even by the antigen binding through αβ TCR combined with the CD4 or CD8 co-receptors. Thus, for activation another co-receptor CD28, has to engage its ligand CD80 or CD86 on the professional antigen-presenting cells (dendritic cells, macrophages, and activated B cells). (Figure 3)
The T cell receptor (TCR) like BCR, is non-covalently associated with a molecular signal transduction complex. This helps in transducing the antigen-binding signal to the interior of the cell.
Pattern recognition receptors
Innate immune receptors recognize pathogen-associated molecular patterns, which consist of recurrent patterns of pathogen molecules. These are known as pattern recognition receptors (PRRS). These patterns expressed by microbes are pathogenic or may be non-pathogenic. Some innate immune receptors can recognize antigens associated with damaged or dead host cells, referred as damage-associated molecular patterns (DAMPS).
Conclusion
The cells of our immune system use receptors to recognize ligands. The B cell receptors and T cell receptors are found only in the adaptive immune system. The B cell receptor (BCR) can make both soluble and membrane-bound forms of an immunoglobulin receptor by sharing the same antigen binding site and are encoded by the same gene. However, unlike BCR, T cell receptors are not secreted in soluble form.
On mature B cells the B-cell coreceptor is expressed as a complex of three transmembrane molecules, i.e., CD19, CD21, and CD81. The T cell receptor (TCR) like BCR, is non-covalently associated with a molecular signal transduction complex. This helps in transducing the antigen-binding signal to the interior of the cell.
Pattern recognition receptors are pathogen-associated molecular patterns that consist of recurrent patterns of pathogen molecules recognized by innate immune receptors.
You may also like:
- Immune system recognition leads to immune responses
- The development of B cell defined by immunoglobulin gene rearrangements
- The external barriers of innate immunity

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around twelve years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.
