In this article, I briefly describe how HIV infects its host by using its coreceptors.
Human Immunodeficiency Virus (HIV)
Acquired immunodeficiency syndrome (AIDS) is a severe immunodeficiency caused by the human immunodeficiency virus (HIV). This syndrome was first recognized because of opportunistic infections in a cluster of individuals in the USA in 1981. This group showed unusual infections with the opportunistic fungal pathogen Pneumocystis jirovecii. This pathogen causes pneumocystis pneumonia in people with immunodeficiency. Previously, people dependent on immunosuppressive drugs had been suffering from the disease. In addition to this disease, patients also suffer from Kaposi’s sarcoma, a very rare skin cancer caused by a virus. A complete analysis showed that all patients had a marked deficiency in cell-mediated immune responses and a significant decrease in the subpopulation of T cells.
After its discovery in the 1980s, AIDS has increased to pandemic proportions throughout the world. The rate of increase in the number of people living with HIV infection has slowed since 2000. The number of new HIV infections peaked in the late 1990s and later slowed down annually.
Retrovirus Causes AIDS
The causative agent of AIDS, known as HIV-1, was discovered and characterized in the laboratories of Luc Montagnier in Paris and Robert Gallo in Bethesda, Maryland. The causative agent, HIV-1, is a retrovirus (Figure 1) of the lentivirus genus. The virus has RNA as its genetic material, and after it enters the host, the RNA is reverse transcribed to DNA by the enzyme reverse transcriptase. This complementary cDNA copy of the viral genome is then integrated into the cell’s chromosomes and replicated along with the cell’s DNA.

The Structure of HIV
The virus has a lipid bilayer envelope with embedded viral proteins that mediate host cell infection. Two copies of a single-stranded RNA genome are inside the virus and associated with a series of proteins, which are important for virus replication. The three structural genes in HIV, such as gag, pol, and env, encode important proteins for the virus (Figure 2). The gag gene encodes many proteins, including the capsid and matrix, which enclose the viral genome and associated proteins. The pol gene encodes three main enzymes: reverse transcriptase, integrase, and protease, which are essential for the viral life cycle. The env gene encodes two surface proteins, gp120 and gp141, responsible for the attachment of the virus to the CD4+ viral receptor and its co-receptor, either CXCR4 or CCR5. The six regulatory genes (tat, rev, nef, vif, vpr, and vpu) in HIV modulate CD4 and MHC class I expression.

HIV Uses Co-receptors to Infect a Target Cell
The human deficiency virus infects cells that express the CD4 protein on their surface, such as TH cells, macrophages, dendritic cells, and brain microglial cells. The high-affinity binding of gp120 to the CD4 molecule on the host cell makes CD4 a favorable attacking point. However, for productive infection virus needs the expression of another co-receptor. The HIV co-receptors CCR5 and CXCR4 are chemokine receptors. These receptors bind chemokines, chemoattractants that guide the migration of cells. The virus’s gp120 protein binds CD4, and this binding brings a conformational change that exposes a binding site for either CXCR4 or CCR5 (Figure 3).

Binding to the co-receptor helps establish contact between the fusion-promoting region of the gp41 protein and the membrane. This triggers the fusion of the lipid bilayer of the virus with the cell membrane. This eventually leads to the release of the contents of the virus into the cytoplasm. The CXCR4 co-receptor helps infect naïve and central memory T cells. The virus gets entry into macrophages and microglial cells as well as effector memory T cells through the CCR5 co-receptor.
Activation of the Provirus
Once the inner part of the HIV enters a cell, its RNA genome is converted into a double-stranded cDNA copy through a two-step process carried out by the enzyme reverse transcriptase. Long terminal repeat (LTR) sequences at both ends help this viral DNA integrate into the host cell’s chromosomes with the assistance of the viral integrase enzyme. This integrated form, the HIV provirus, undergoes transcription, producing viral RNA that is then spliced and translated into proteins.
These proteins, along with newly synthesized full-length copies of the HIV genome, come together to form new virus particles (Figure 4). The viral protease enzyme breaks some larger viral protein precursors into smaller proteins that constitute the nuclear capsid and the three key enzymes in mature viruses. Meanwhile, the gp120 and gp41 envelope proteins, initially formed as a single precursor in the endoplasmic reticulum, are cleaved and transported to the plasma membrane. At the membrane, other viral components, including the proteins and RNA genome, assemble, and new HIV virions are released as they bud from the infected cell’s surface.
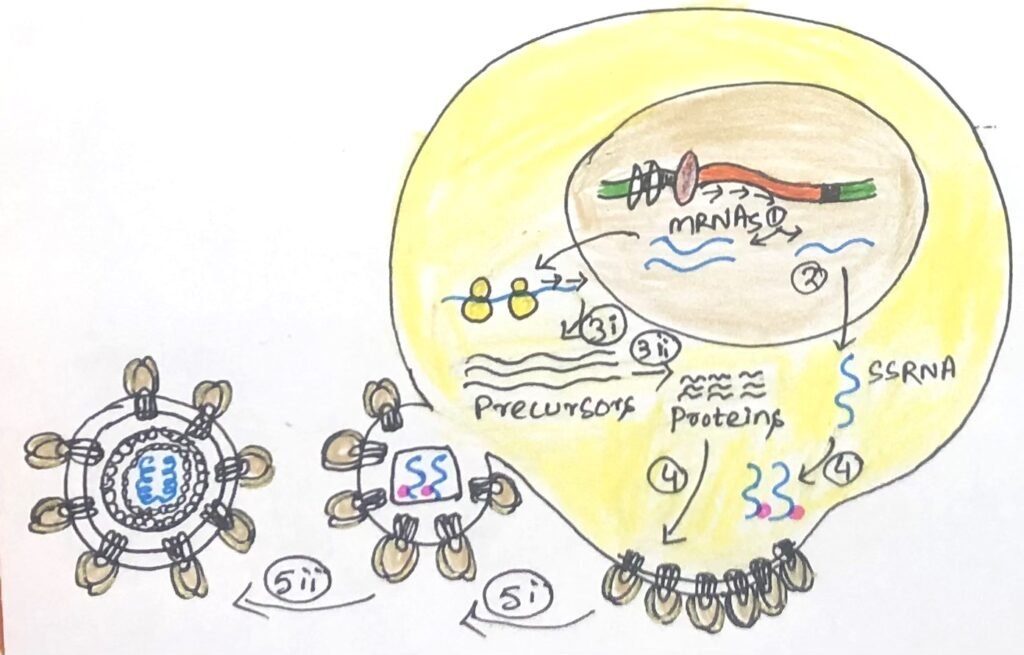
The active transcription of the viral genome causes a large number of viral particles to bud off from the plasma membrane. Too much engagement of the host cell’s biosynthetic machinery and plasma membrane in virus production can cause cell lysis. HIV can become dormant for long periods in an infected cell. This dormancy period imposes a heavy task on the immune system to search and eliminate these latently infected cells. Latent infection leads to the formation of HIV reservoirs, which serve as protective shelters for the virus, making it resistant to both the body’s immune response and antiretroviral treatments.
HIV Variants Favoring CCR5 or CXCR4 Co-receptors Have Distinct Roles in Infection
The human deficiency virus (HIV) uses CCR5 and CXCR4 as its major co-receptors. Effector memory CD4+ T cells, macrophages, brain microglial cells, and dendritic cells express CCR5. Naïve CD4+ T cells and central memory T cells express CXCR4. The variants of HIV that bind the CCR5 co-receptor are called R5 variants, which infect macrophages, dendritic cells, and effector memory T cells. The X4 variants preferentially infect naïve and central memory T cells (Figure 5).

In vitro studies have shown that certain chemokines, like RANTES, can inhibit virus replication. Since CCR5 and CXCR4 cannot bind to both HIV and their natural chemokine ligands at the same time, competition for these receptors between the virus and the chemokines can prevent the virus from entering the host cell.
R5 Viruses and X4 Viruses Act Differently in HIV Infection
The two HIV variants, R5 and X4, play different roles in HIV infection. During intercourse, most of the CD4+ cells in the epithelial layers are exposed to virus-containing body fluids. Generally, the R5 viruses are responsible for the initial infection. As epithelial tissues face constant microbial exposure, they have many macrophages, dendritic cells, and T cells. Additionally, some epithelial cells express CCR5, which may facilitate the virus’s transcytosis through the epithelial barrier.
The CCR5 co-receptor is expressed on the long extensions of dendritic cells between epithelial cells. It pulls viruses from the surface into the epithelial layer. Then R5 variants are either delivered to CD4+ T cells in the epithelium or carried to the draining lymph nodes. Thus, the R5 viruses seem to be the initial infecting virus that is responsible for dissemination to other lymphoid tissues throughout the body. The X4 variants arise late in the course of HIV infection and eliminate a plethora of T cells. This causes AIDS. The virus binds to the CCR5 or CXCR4 co-receptor by studying the viral envelope protein gp120.
CCR5 Mutation: A Path to HIV Resistance and a Potential Cure
A deletion mutation in the CCR5 gene provides almost complete resistance to infection by the R5 strains of HIV, which are the primary variants transmitted through sexual contact. Individuals who inherit this mutation from both parents (homozygous) lack CCR5 on their cell surfaces, rendering them resistant to HIV strains that depend on this co-receptor. Interestingly, the absence of CCR5 does not appear to cause any significant adverse effects. This discovery has sparked new ideas and optimism for HIV eradication.
One notable case is Timothy Ray Brown, also known as the Berlin Patient. In 2007, he underwent a bone marrow transplant to treat leukemia, receiving stem cells from a donor who carried the CCR5 mutation. As a result, he has remained free of the virus for over a decade without the need for antiretroviral therapy. To date, he is the only documented HIV-positive individual to have seemingly achieved a complete cure.
Conclusion
Acquired immunodeficiency syndrome (AIDS) is a severe immunodeficiency caused by the human immunodeficiency virus (HIV). The causative agent, HIV-1, is a retrovirus of the lentivirus genus. The virus has RNA as its genetic material. When it enters the host, the RNA is reverse transcribed to DNA by the enzyme reverse transcriptase. This complementary cDNA copy of the viral genome is then integrated into the cell’s chromosomes, where it is replicated along with the cell’s DNA.
The three structural genes in HIV, such as gag, pol, and env, encode important proteins for the virus. The env gene encodes two surface proteins, gp120 and gp141, which are responsible for the attachment of the virus to the CD4+ viral receptor and its co-receptor, either CXCR4 or CCR5. The virus’s gp120 protein binds CD4. This binding brings a conformational change that exposes a binding site for either CXCR4 or CCR5. Binding to the co-receptor helps establish contact between the fusion-promoting region of the gp41 protein and the membrane.
The two HIV variants, R5 and X4, play different roles in HIV infection. During intercourse, most of the CD4+ cells in the epithelial layers are exposed to virus-containing body fluids. The R5 viruses are responsible for the initial infection. As epithelial tissues face constant microbial exposure, they have many macrophages, dendritic cells, and T cells. CCR5 is expressed by some epithelial cells and may ease the transcytosis of the virus through the epithelial layer. A deletion mutation in the CCR5 gene provides almost complete resistance to infection by the R5 strains of HIV, which are the primary variants transmitted through sexual contact.
You may also like:
- Breakthrough treatments of HIV based on the protease mechanism
- Various factors cause secondary immunodeficiencies
- Viruses as Hidden Causes of Cancer

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.




