In this article, I briefly explain the precipitation reaction, which is a type of antigen-antibody interaction.
Antigen-antibody interaction
The Interaction between antigen and antibody is a bimolecular association, which does not lead to an irreversible chemical alteration in either the antibody or the antigen. The antigen-antibody association involves many non-covalent interactions between the antigenic determinant (epitope) of the antigen and the variable-region (VH/VL) domain of the antibody molecule. The antigen-antibody binding depends on weak and non-covalent interactions like hydrogen bonds, hydrophobic interactions, electrostatic forces, and Van der Walls interactions. Thus, to make a sturdy antigen-antibody interaction, a large number of such weak interactions are required. These interactions can only take place if the antigen and antibody molecules are close enough for some of the individual atoms to fit into complementary recesses.
Properties of antigen-antibody interaction
Affinity and avidity
The strength of antigen-antibody interaction depends on a very close fit between the two. Affinity is the sum of the attractive and repulsive forces operating between the antigenic determinant and the combining site of the antibody. The affinity of an antibody for a specific epitope is the combined strength of the non-covalent interactions between a single antigen-binding site on an antibody and the epitope.
The strength of multiple interactions between a multivalent antibody and antigen is called avidity. When complex antigens containing multiple repeating antigenic determinants are mixed up with antibodies containing multiple binding sites, the interaction of an antibody with an antigen at one site will increase the probability of a reaction between those two molecules at a second site. Avidity is more than the sum of the individual affinities. Affinity defines the strength of interaction between antibody and antigen at single antigenic sites, whereas avidity defines the overall stability or strength of the antibody-antigen complex. The strength of the antibody-antigen complex is controlled by three major factors. The factors include the antibody-epitope affinity, the valence of both the antigen and antibody and the structural arrangement of the interacting parts.
Specificity and cross-reactivity
The specificity of an antigen-antibody reaction is the ability of an individual antibody combining site to react with only one antigenic determinant. It also defines the ability of a population of antibody molecules to react with only one antigen. An antibody can interact with its antigen, thus making the antigen-antibody reactions highly specific. A strong antigen-antibody interaction depends on a very close fit between the antigen and antibody, which requires a high degree of specificity.
Cross-reactivity
Though the antigen–antibody interaction is highly specific, sometimes the antibody elicited by one antigen can cross-react with an unrelated antigen, called cross-reactivity. Cross-reactions arise because the cross-reacting antigen has an epitope, which is structurally similar to one on the immunizing antigen.
Cross-reactivity is often observed among polysaccharide antigens that contain similar oligosaccharide residues. The glycoproteins expressed on red blood cells are the ABO blood group antigens. Subtle differences in the terminal residues of the sugars attached to these surface proteins distinguish the A and B blood group antigens. An individual lacking one or both of these antigens will have serum antibodies to the missing antigens. Thus, individuals belonging to blood groups O and B have anti-A antibodies in their serum. Similarly, individuals belonging to blood groups O and A have anti-B antibodies in their serum.
Individuals belonging to blood group AB, are believed not to have anti-A nor anti-B antibodies because they express both antigens on their red cells. The antibodies are induced by exposure to cross-reacting microbial antigens present on common intestinal bacteria. Microbial antigens elicit the blood group antibodies, which will cross react with similar oligosaccharides present on foreign red blood cells providing the basis for blood typing tests, and accounting for the necessity of compatible blood types during blood transfusions. A number of viral and bacterial antigens elicit antibody that cross reacts with the host-cell components, which results in a tissue damaging reaction. Some vaccines also exhibit cross-reactivity.
Types of antigen-antibody interaction
There are mainly six types of antigen-antibody interaction and can be categorized as
- Precipitation reaction
- Agglutination reaction
- Complement fixation
- Immunoflorescence
- ELISA- Enzyme linked immunosorbent assay
- Radioimmunoassay (RIA)
Precipitation reaction
A soluble antibody reacts with a soluble antigen to give an insoluble product, which is also known as precipitate and the reaction is known as precipitation reaction. Soluble antibodies that aggregate soluble antigens are called precipitins. Soluble antigen that induces the formation of a specific precipitin, is called a precipitinogen.
When antigens having at least two epitopes per molecule are cross-linked by the bivalent antibodies, it results in the formation of a lattice. This lattice ultimately develops into a visible precipitate. For the lattice to be formed, the bivalent antibody will bind to epitopes on two different antigens. A complex is formed when a second antibody molecule combines with the second epitope on one of the antigen molecules and a third epitope on another antigen molecule. The complex continues to grow and when it is large enough in size, becomes insoluble and can be visible as a precipitate. Thus, formation of the visible precipitate takes much longer time than formation of the soluble antigen-antibody complexes.
Precipitation reaction can occur using polyclonal antibodies or mixture of monoclonal antibodies. Large aggregates are formed by polyclonal antibodies that precipitate out of the solution. If case of a monovalent antigen, or a single monoclonal antibody, the antibody can link only two molecules of antigen. This results in the formation of no precipitate. Precipitation reaction depends on the concentration of the antigen. The reaction is affected by changing the concentration of the antigen.
The three zones by the precipitation curve
A quantitative precipitation reaction can be performed by placing a constant amount of antibody in a series of tubes, and adding increasing amounts of antigens to the tubes. When a plot is made with the amount of precipitate against increasing antigen concentrations, it yields a precipitation curve. For a system of antigen-antibody, the precipitation curve shows (figure 1) three zones. The first zone is the zone of antibody excess or prozone in which the antigen concentration is very low and that of the antibody is relatively high, as a result precipitation is inhibited. Thus, formation of small complexes occur, and residual antibodies will remain in the supernatant.
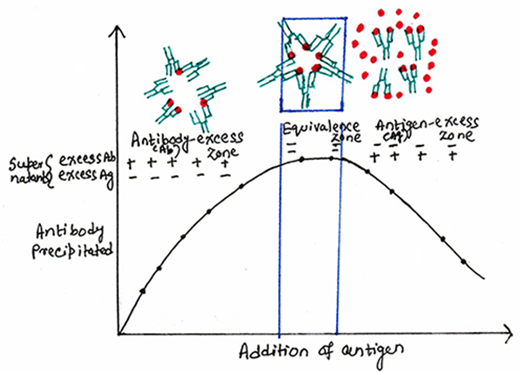
The second zone is called the zone of equivalence, or the zone of maximal precipitation in which antigen and antibody form large insoluble complexes. In this zone, the absence of antigen and antibody is marked in the supernatant. The third zone is the zone of antigen excess or postzone in which the antigen concentration is very high. Thus, with increasing the amount of antigen, the lattice size becomes too small to precipitate. As a result, precipitation is inhibited and antigen-antibody binding is absent in the supernatant.
Types of precipitation reactions
Antigen-antibody forms a precipitate in both solution and gel. In both fluid and gel, visible precipitation occurs in the region of equivalence. In regions of antibody or antigen in excess, no visible precipitate is formed.
Fluid phase precipitation
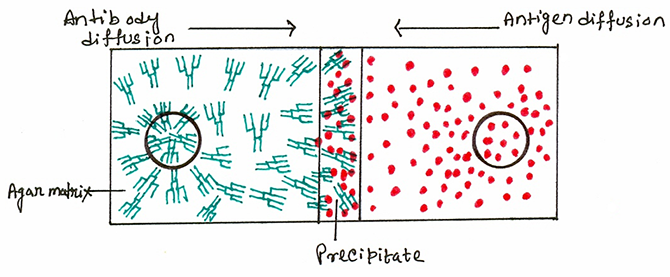
This method is used to identify unknown antigen or unknown antibody. It involves the layering of an antigen solution over an antibody solution in a capillary tube. In this double diffusion method, both antigen and antibody will diffuse toward each other. At the interface, antibody recognizes antigen, leading to the formation of precipitate. The amount of the precipitate is proportional to the concentration of both antigen and antibody.
Gel phase precipitation
In this method of precipitation, instead of solution, gel is used as a semisolid medium. The gel contains “pores” that allow the movement of molecules. The gel is a derivative of agar and is called agarose. Agarose gel allows soluble antigen and /or antibody to diffuse through the pores until they reach the optimal concentration for lattice formation. Smaller molecules move through the gel faster than larger molecules.
When antibody is incorporated into the agar, and antigen diffuses into the antibody-containing matrix, a visible line of precipitation is observed, which is unlike the precipitation curve in fluids. The same is also observed when antigen and antibody diffuse toward one another in agar. Two types of immunodiffusion reactions can be used to determine relative concentrations of antibodies or antigens, i.e., radial immuno-diffusion and double immuno-diffusion.
Radial immunodiffusion ( The Mancini method)
In this method, an antigen sample is placed in a well and allowed to diffuse into agar containing a suitable dilution of an antiserum (figure 2). The antigen diffuses in all directions from the well. Thus, the region of equivalence is established, and a ring of precipitation (precipitin ring) forms around the well. The area of the precipitin ring is proportional to the concentration of antigen. To determine the antigen concentration, the diameter of the area of precipitation, along with the well diameter, is measured.

Double immunodiffusion (The Ouchterlony method)
This method involves the detection of antigen by placing a known reagent antibody in the center well and the unknown samples in the surrounding wells. Similarly to detect an antibody, the known antigen is placed in the center well (figure 3). After each of the samples and reagents have been added to the appropriate wells, diffusion occurs. Both antigen and antibody diffuse radially from wells toward each other, thereby establishing a concentration gradient. A line of precipitation forms at the zone of equivalence.
Conclusion
The antigen-antibody bonding depends on weak and non-covalent interactions. These interactions include hydrogen bonds, hydrophobic interactions, electrostatic forces, and Van der Walls interactions.
The affinity of an antibody for a specific epitope is the combined strength of the non-covalent interactions between a single antigen-binding site on an antibody and the epitope. The strength of multiple interactions between a multivalent antibody and antigen is called the avidity. A strong antigen-antibody interaction depends on a very close fit between the antigen and antibody, which requires high degree of specificity. The antigen–antibody interaction is highly specific. However, sometimes the antibody elicited by one antigen can cross-react with an unrelated antigen, known as cross-reactivity.
A soluble antibody reacts with a soluble antigen to give an insoluble product. This insoluble product is known as precipitate, and the reaction is known as precipitation reaction. There are three types of precipitation reactions, i.e., fluid phase precipitation, gel phase precipitation, and radial immunodiffusion. Fluid phase precipitation is used to identify unknown antigen or unknown antibody. In gel-phase precipitation instead of solution, gel is used as a semisolid medium. The gel contains “pores” that allow the movement of molecules. In double immunodiffusion, identification and quantification of antigens and antibodies are determined by the process of diffusion.
You may also like:
- The antigen-antibody interaction: Agglutination reaction
- The antigen-antibody interaction- Enzyme-linked Immunosorbent Assay (ELISA)
- The antigen-antibody interaction- Immunofluorescence

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.



