In this article, I briefly describe the radioimmunoassay, which is a process of antigen-antibody interaction.
Antigen-antibody interaction
The Interaction between antigen and antibody is a bimolecular association, which does not lead to an irreversible chemical alteration in either the antibody or the antigen. The antigen-antibody association involves many non-covalent interactions between the antigenic determinant (epitope) of the antigen and the variable-region (VH/VL) domain of the antibody molecule. The antigen-antibody binding depends on weak and non-covalent interactions like hydrogen bonds, hydrophobic interactions, electrostatic forces, and Van der Walls interactions. Thus, to make a sturdy antigen-antibody interaction, many such weak interactions are required. These interactions can only occur if the antigen and antibody molecules are close enough for some individual atoms to fit into complementary recesses.
Affinity and avidity
A very close fit between antigen and antibody increases the strength of the bond. The sum of the attractive and repulsive forces operating between the antigenic determinant and the combining site of the antibody determines the affinity. The affinity of an antibody for a specific epitope is the combined strength of the non-covalent interactions between a single antigen-binding site on an antibody and the epitope.
The strength of multiple interactions between a multivalent antibody and antigen is called avidity. When complex antigens containing multiple repeating antigenic determinants are mixed up with antibodies containing multiple binding sites, the interaction of an antibody with an antigen at one site will increase the probability of a reaction between those two molecules at a second site. Avidity is more than the sum of the individual affinities. Affinity defines the strength of interaction between antibody and antigen at single antigenic sites, whereas avidity defines the overall stability or strength of the antibody-antigen complex. The strength of the antibody-antigen complex is controlled by three major factors, i.e., antibody-epitope affinity, the valence of both the antigen and antibody and the structural arrangement of the interacting parts.
Specificity and cross-reactivity
The specificity of an antigen-antibody reaction is the ability of an individual antibody combining site to react with only one antigenic determinant. It also defines the ability of a population of antibody molecules to react with only one antigen. An antibody can interact with its antigen, thus making the antigen-antibody reactions highly specific. A strong antigen-antibody interaction depends on a very close fit between the antigen and antibody, which requires a high degree of specificity.
Sometimes, the antibody elicited by one antigen can cross-react with an unrelated antigen, called cross-reactivity. The cross-reacting antigen has an epitope, which is structurally similar to one on the immunizing antigen.
Types of antigen-antibody interaction
There are mainly six types of antigen-antibody interaction and can be categorized as
- Precipitation reaction
- Agglutination reaction
- Complement fixation
- Immunofluorescence
- ELISA- Enzyme-linked immunosorbent assay
- Radioimmunoassay (RIA)
Radioimmunoassay
This sensitive technique was first developed in 1960 by endocrinologists, S.A. Berson and Rosalyn Yalow. It is an in vitro assay technique, which is used for the separation of a protein from a mixture. The protein is separated using the specificity of antibody-antigen binding and quantitation using radioactivity. S.A. Berson and Rosalyn Yalow used this test to determine levels of insulin-anti-insulin complexes in diabetics. The technique is extremely sensitive and specific. Though it requires specialized equipment, it is the least expensive method to perform such tests. It requires special precautions and licensing since radioactive substances are used.
Principle of RIA
The principle of RIA involves competitive binding of radiolabeled antigens and unlabeled antigens to a high-affinity antibody. At first, a known quantity of antigen is made radioactive. Thus, it is labeled with gamma-radioactive isotopes of iodine attached to tyrosine. The labeled antigen is then mixed with the antibody at a concentration that saturates the antigen-binding sites of the antibody. Then, test samples of unlabeled antigens of unknown concentration are added in progressively larger amounts. The test sample containing the unlabeled antigen may be a complex mixture, such as serum or body fluids. Thus, the unlabeled antigen competes with the radio-labeled antigen for antibody binding sites (figure 1).
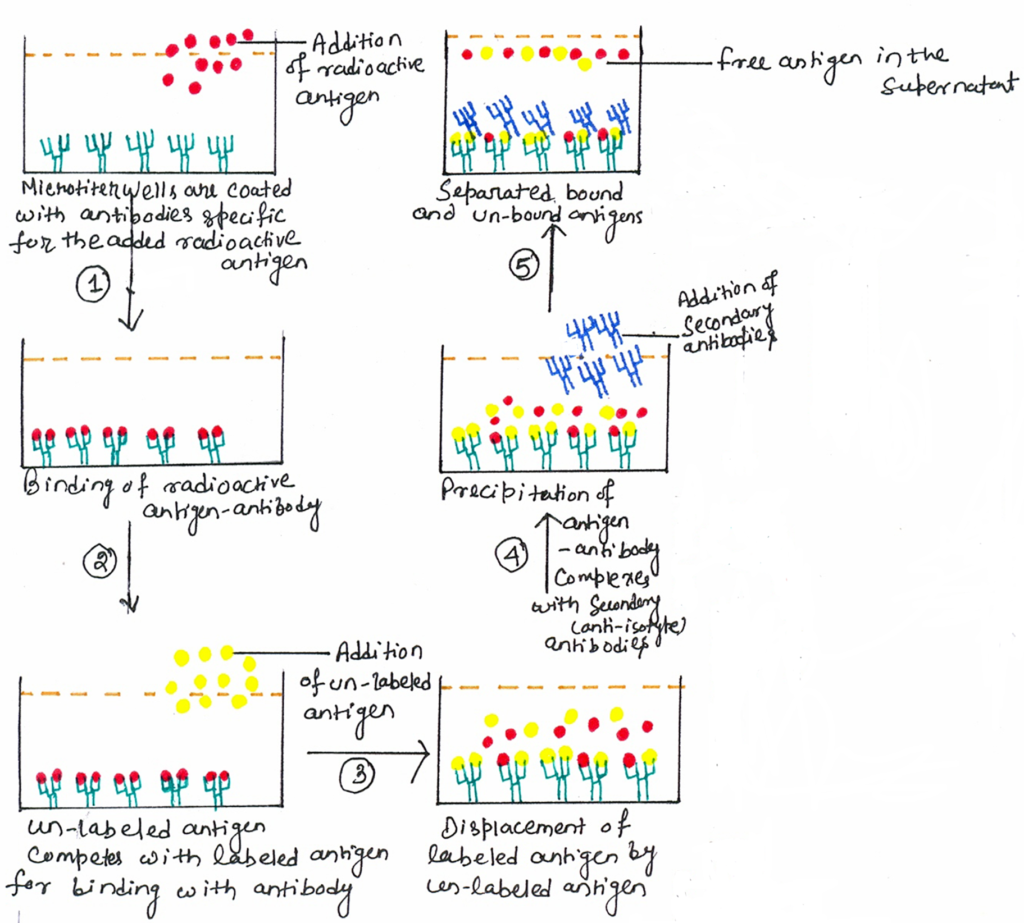
The increase in the concentration of unlabeled antigens will cause the displacement of more labeled antigens from the binding sites. The antigen-antibody complex is precipitated with a secondary anti-isotype antiserum, to separate the bound antigens from the unbound ones. For example, if the antigen-antibody complex contains rabbit IgG antibodies, it is precipitated with goat anti-rabbit IgG, which will bind to the rabbit IgG and precipitate the complex. After the separation process, the radioactivity of the free antigen remaining in the supernatant is measured using a gamma counter.
A binding curve can be generated using known standards, which allows the amount of antigen in the test sample to be derived. A standard curve is obtained by adding increasing concentrations of unlabeled antigens to a fixed quantity of radio-labeled antigens and specific antibodies (figure 2).

The plot of the percentage of labeled antigen bound versus the concentration of unlabeled antigen shows a curve. The concentration of antigen in unknown serum samples can be known by using the linear part of the curve.
Conclusion
The antigen-antibody interaction is a bimolecular association. The antigen-antibody binding depends on weak and non-covalent interactions like hydrogen bonds, hydrophobic interactions, electrostatic forces, and Van der Walls interactions. The affinity of an antibody for a specific epitope is the combined strength of the non-covalent interactions between a single antigen-binding site on an antibody and the epitope.
Radioimmunoassay was first developed in 1960 by endocrinologists S.A. Berson and Rosalyn Yalow. It is an in vitro assay technique, which is used for the separation of a protein from a mixture. It is an extremely specific and sensitive technique. The principle of RIA involves competitive binding of radiolabeled antigens and unlabeled antigens to a high-affinity antibody.
You may also like:

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around twelve years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.
