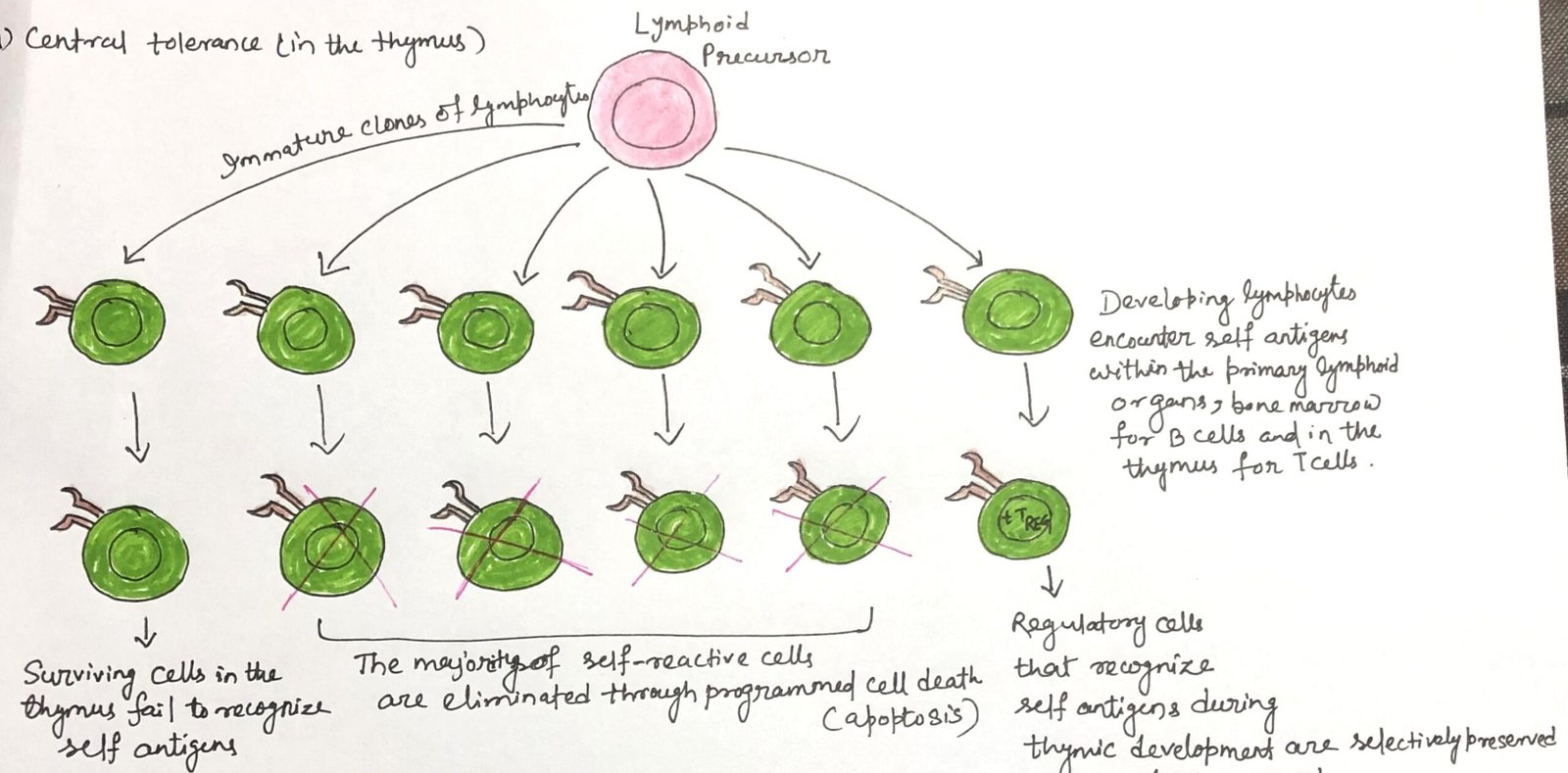
In this article, I briefly describe the central tolerance in primary lymphoid organs. Central tolerance is a fundamental immunological mechanism that ensures self-tolerance by regulating lymphocyte development. It is established within primary lymphoid organs, where self-reactive T and B lymphocytes are eliminated, modified, or redirected into regulatory pathways. This process is essential for maintaining immune homeostasis and preventing autoimmunity.
Immune Tolerance and Antigen Sequestration
Immune tolerance refers to the coordinated mechanisms by which the immune system prevents its cells and antibodies from reacting against the body’s own components. Current understanding emphasizes that tolerance is not a passive absence of response but an active, highly regulated process. Immune cells continuously recognize self-antigens and beneficial commensal microbes, thereby preserving tissue integrity while maintaining immune balance.
One important mechanism that limits self-reactivity is antigen sequestration, in which certain self-antigens are physically isolated from immune surveillance. Structures such as the anterior chamber and lens of the eye are considered immune-privileged sites due to minimal lymphatic drainage. As a result, tissue-specific antigens in these regions have restricted contact with immune cells, reducing the likelihood of immune-mediated damage under normal conditions.
However, sequestration also has limitations. Because these antigens are largely excluded from immune tolerance pathways, their sudden exposure following tissue injury may trigger an aggressive immune response. This phenomenon is evident in ocular trauma, where breach of immune privilege can result in inflammation, tissue damage, and loss of function. Thus, antigen sequestration represents a protective but context-dependent mechanism of immune tolerance.
Central Tolerance and the Elimination of Self-Reactive Lymphocytes
Central tolerance is a regulatory process operating in the primary lymphoid organs that ensures immune self-recognition by selectively eliminating or shaping self-reactive lymphocytes. During lymphocyte development, mechanisms that generate antigen receptor diversity, such as DNA rearrangement of variable gene segments and the random addition of nucleotides at gene junctions, inevitably produce T-cell and B-cell receptors capable of recognizing self-antigens.
Induction of Central Tolerance in Primary Lymphoid Organs
If all such self-reactive lymphocytes were permitted to mature and enter the peripheral immune system, autoimmune disorders would be frequent. To prevent this outcome, developing T and B lymphocytes that bind strongly to self-antigens are removed during early stages of maturation in the thymus (Figure 1) and bone marrow. This elimination mainly occurs through apoptosis of cells expressing high-affinity T-cell or B-cell receptors specific for antigens in these organs. Through this selective deletion, central tolerance establishes a self-tolerant immune repertoire before lymphocytes reach functional maturity.
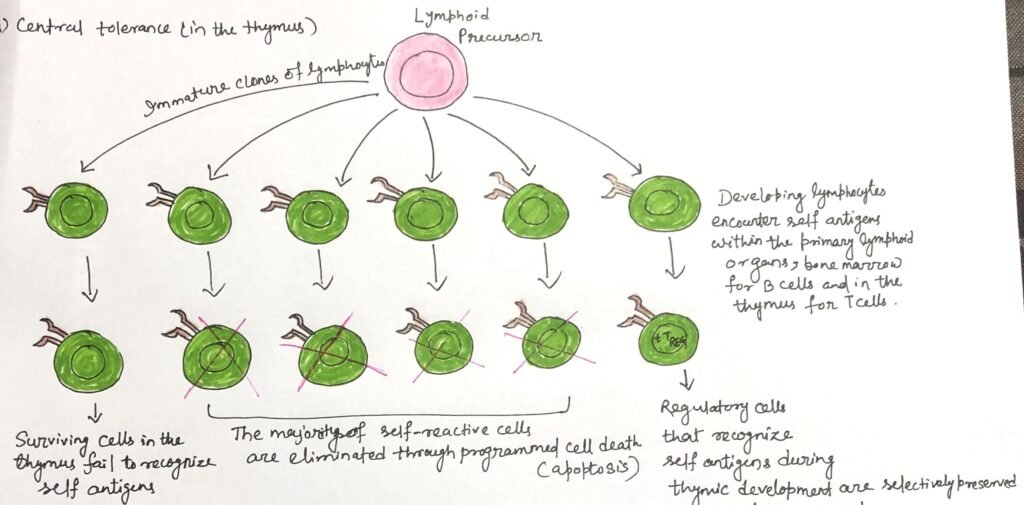
This elimination process is known as negative selection. It induces programmed cell death in developing lymphocytes that express high-affinity T-cell or B-cell receptors for self-antigens present in primary lymphoid organs. In the thymus, the transcription factor AIRE promotes the expression of tissue-specific self-antigens by medullary thymic epithelial cells.
The presentation of these antigens allows for the efficient deletion of potentially harmful self-reactive T cells before they reach maturity. Disruption of this regulatory pathway can have serious consequences. These can be defects or mutations in the AIRE gene, as well as in other tolerance-controlling mechanisms. This can impair central tolerance and is associated with the development of severe, multisystem autoimmune disorders. Through negative selection and AIRE-dependent antigen expression, central tolerance plays a critical role in preventing widespread autoimmunity.
Mechanisms of Central Tolerance
Studies by David Nemazee and colleagues revealed that developing B cells use receptor editing as an additional central tolerance safeguard. Instead of being deleted, some self-reactive B cells can change their antigen specificity through additional V–J rearrangements at the light-chain loci. This process replaces the original variable region with a new one, often generating a receptor. The receptor has reduced or absent self-reactivity, allowing the cell to survive.
Receptor editing and negative selection are essential mechanisms of central tolerance. Together, they limit the emergence of autoreactive lymphocytes during early development in primary lymphoid organs. Although most self-reactive cells are eliminated, a few may leave the thymus or bone marrow in an anergic state. These cells are subsequently eliminated in the peripheral tissues through apoptotic pathways, providing an additional layer of immune self-regulation.
Regulatory T Cells and Their Role in Central Tolerance
During central tolerance, not all lymphocytes that recognize self-antigens are eliminated. A subset of developing T lymphocytes in the thymus is selectively preserved and differentiates into regulatory T cells (TREG cells). These cells are characterized by the expression of the transcription factor FoxP3, which is essential for their development and function. After migrating from the thymus, regulatory T cells help maintain immune stability. They control harmful immune responses against self-antigens in peripheral tissues.
Regulatory T cells often carry T-cell receptors that recognize self-antigens with high affinity, similar to lymphocytes typically marked for deletion. The fate of a self-reactive T cell, deletion or differentiation into a regulatory T cell, depends on multiple influencing factors. These factors include CD28 binding to CD80/86 and CD40 interacting with CD40L, as well as the surrounding cytokine environment.
Current models suggest that the nature of TCR signaling also plays a decisive role. Brief, high-affinity but transient interactions between TCRs and antigen-presenting molecules in the thymic medulla are thought to promote the formation of regulatory T cells. In contrast, prolonged and strong TCR engagement generally leads to the elimination of self-reactive lymphocytes through apoptotic pathways.
Thymus-derived regulatory T cells (tTregs) that complete their development migrate into peripheral tissues, where they function to suppress immune responses directed against self-antigens. Among these cells, CD4⁺ tTREG cells constitute the dominant population produced in the thymus. Whereas CD8⁺ thymus-derived regulatory T cells appear to be comparatively rare.
Conclusion
Central tolerance is a fundamental process that safeguards the body from autoimmune reactions. It shapes the developing lymphocyte repertoire within primary lymphoid organs, namely the thymus and bone marrow. Through mechanisms such as negative selection, receptor editing, and the induction of apoptosis, most self-reactive T and B lymphocytes are eliminated before reaching maturity. In addition, transcription factors like AIRE enhance tolerance by promoting the expression of tissue-specific antigens in the thymus. This enables the removal of potentially harmful autoreactive T cells. Not all self-reactive lymphocytes are deleted, but some differentiate into regulatory T cells that actively suppress immune responses against self-antigens in peripheral tissues. Although certain autoreactive cells may escape central tolerance, they are often rendered functionally inactive or removed later through peripheral regulatory mechanisms. Together, these coordinated processes ensure immune self-recognition, maintain immune homeostasis, and prevent the development of autoimmune diseases.
You may also like:
- Immune response gets started in the secondary lymphoid organs
- The effector cells of the immune system
- Immunodeficiency Disorders Can Lead to the Development of Autoimmunity

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.