In this article, I briefly describe various replacement therapies that treat immunodeficiency disorders.
Treatment For Immunodeficiencies
There are many treatment options available for immunodeficiency disorders, but no reliable treatment is available for them. Besides using antimicrobial agents or resorting to the extreme measure of complete isolation from opportunistic pathogens, immunodeficiencies can be managed through replacement therapy that restores missing proteins, cells, or genes.
Gene Replacement Therapy
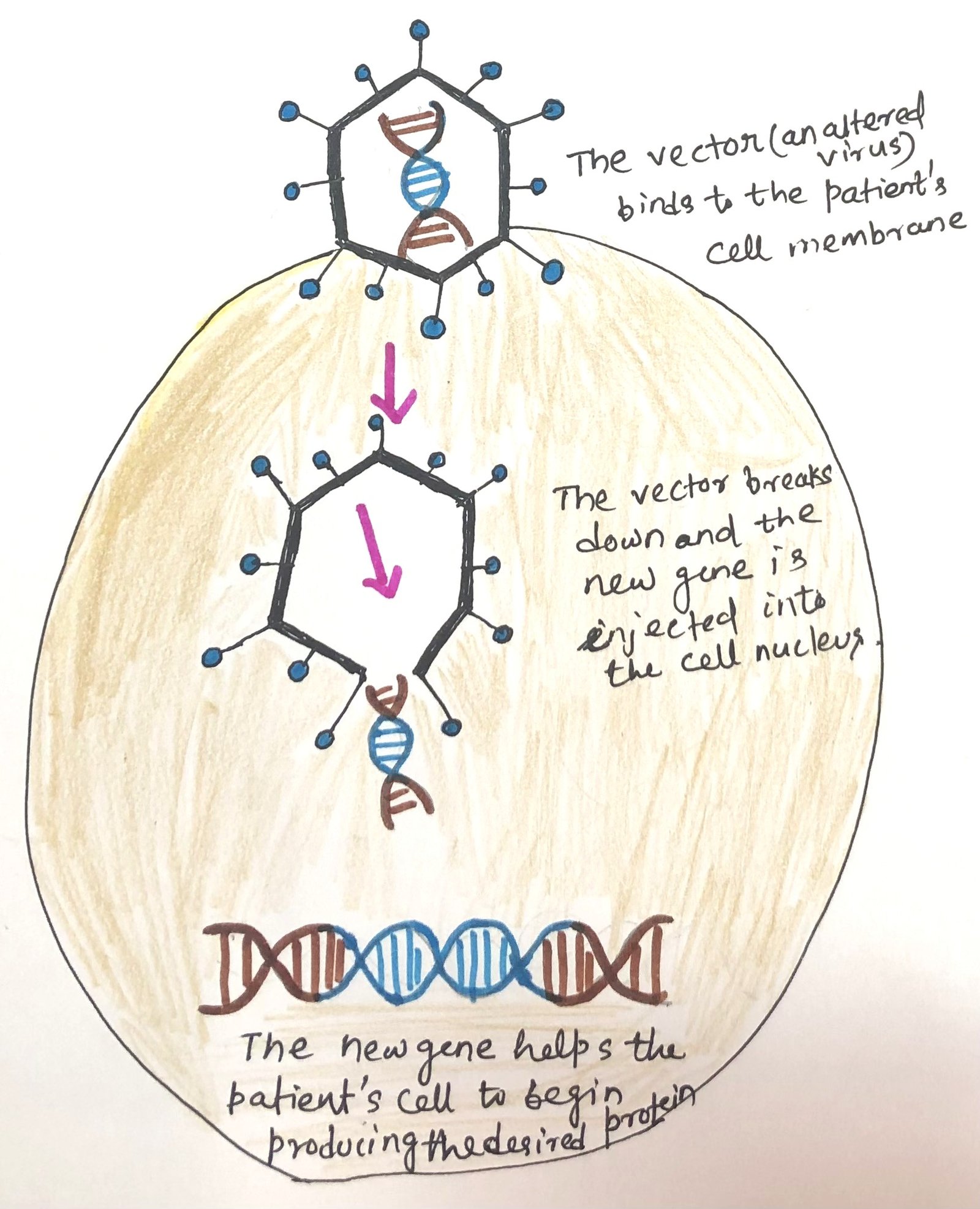
The process of gene replacement therapy treats some immunodeficiency diseases. In case of a single gene defect, like in adenosine deaminase (ADA) or common gamma chain (SCIDX1) defects, gene replacement therapy can be applied. The single defective gene can be replaced as a treatment option (Figure 1). Replacement gene therapy aims to substitute non-functional genes with functional ones, to provide substantial benefits or even potential cures. This approach involves encasing healthy genes within modified viruses—typically mild adeno-associated viruses—which serve as carriers. These vectors are administered in a single dose, through the bloodstream or directly to the affected area. The altered viruses are engineered to bind to specific cells and transport the new genes into their nuclei, accompanied by promoters that stimulate the genes to instruct cells to produce essential proteins that are otherwise deficient or nonfunctional.

Clinical trials for the two types of SCID (severe combined immunodeficiency) have been carried out. These trials involve the isolation of CD34+ hematopoietic stem cells (HSCs) from the bone marrow or umbilical cord blood of HLA-identical or haploidentical donors. A retroviral vector helps these cells be transduced with the corrected gene. This leads to the insertion of a copy of the gene into the cells’ chromosomes. Then, they are introduced into the patient.
Gene Therapy Helps to Reconstitute T cells and B cells
Gene therapy has been quite successful for the ADA (adenosine deaminase) deficiency SCID. Patients with ADA-SCID receiving gene therapy have significant long-lasting reconstitution of their T cells and B cells. These patients are also able to enhance their immune responses. The first twenty babies with SCIDX1 around the world received gene therapy. Out of the twenty children, eighteen are alive, and seventeen children have successfully restored the development of T lymphocytes and immune function. However, five of these SCID-free patients developed leukemia. It was due to the insertion of the transferred gene in the vicinity of oncogenes. Four of the five children were cured of leukemia, while one child succumbed to the disease. A more promising approach has recently been developed with a vector from lentivirus, a retrovirus. Patients with SCIDX1 who received this vector had effective reconstitution of B, T, and NK cells with no toxicity.
Gene Therapy Helps Correct Defective Genes in Stem Cells
A novel and promising approach in gene therapy involves correcting defective genes within a patient’s hematopoietic stem cells (HSCs), either in vitro or directly in vivo. This method utilizes the advanced CRISPR/Cas9 gene-editing technology to replace mutated DNA sequences with normal ones. Recently, CRISPR/Cas9 has been successfully employed to correct a defective gene in hematopoietic stem cells (HSCs) from a patient with a specific type of chronic granulomatous disease. When these modified HSCs were transplanted into immunodeficient mice, they effectively produced functional myeloid and lymphoid cells, demonstrating the potential of this approach in treating genetic disorders.
Cell Replacement Therapy
Progress in bone marrow and hematopoietic stem cell (HSC) transplantation has enabled cell replacement therapy for certain immunodeficiencies. Currently, this remains the primary long-term treatment option for patients with severe combined immunodeficiency (SCID). Transplanting cell populations containing HSCs from an immunocompetent donor facilitates the development of a functional immune system in the recipient.
The rate of success depends on the timing of transplantations. Success chances are highest when the transplantations are done in the first 3.5 months of life before infection. For recipients who get an HLA-identical donor, especially a sibling, the survival chances are also greater. These procedures can also be relatively successful in infants with SCID when haploidentical donor bone marrow is used. Here, T cells are depleted to avoid graft-versus-host reactions, where donor-derived T cells attack the recipient, a major adverse complication of HSC transplantation.
Hematopoietic Stem Cell Transplantation
Hematopoietic stem cell (HSC) transplantation is still developing as a broad treatment for immunodeficiencies due to the complexity of blood cell formation, the diverse effects of immunodeficiencies, the necessity of restoring all blood cell lineages, and the risk of graft-versus-host disease. The ability of donor hematopoietic stem cells (HSCs) to establish themselves in the recipient is limited when there is insufficient space in the bone marrow and thymus for their expansion and differentiation. To address this, many recipients undergo myeloablative conditioning before transplantation. This treatment depletes existing HSCs and bone marrow cells, creating room for donor-derived HSCs, progenitor cells, and precursor cells.
However, the drugs used in this process have side effects, as they are inherently toxic to cells. Moreover, not all blood cell types regenerate with the same efficiency after transplantation. For instance, haploidentical HSC transplantation in infants with X-linked severe combined immunodeficiency (SCID) results in over 70% long-term survival. Yet, in about two-thirds of these patients, while T cells successfully engraft and become functional within a few months, B cells and natural killer (NK) cells rarely develop. As a result, these patients require continued intravenous immunoglobulin (IVIG) therapy.
Protein Replacement Therapy
Some disorders hinder antibody production. This condition is treated by the administration of the missing immunoglobulins. In various kinds of immunodeficiency, the injection of pooled human gammaglobulin, known as intravenous immunoglobulin (IVIG), protects against recurrent infections.
Advancements in the development of human monoclonal antibodies and the genetic engineering of chimeric antibodies, which combine mouse-derived V regions with human C regions, have enabled the production of injectable antibodies tailored to target significant pathogens.
To produce large quantities of purified proteins for treating deficiencies in enzymes, cytokines, and complement components, genes can be expressed in vitro using bacterial or eukaryotic expression systems. These proteins enable novel therapeutic approaches by replacing or increasing the levels of immunologically important proteins in patients. For instance, recombinant adenosine deaminase has been successfully used to treat ADA-deficient SCID, while recombinant IFN-gamma has shown effectiveness in managing chronic granulomatous disease.
Conclusion
There are many treatment options available for immunodeficiency disorders. Immunodeficiencies can be managed through replacement therapy that restores missing proteins, cells, or genes. The process of gene therapy treats some immunodeficiency diseases. In case of a single gene defect, like in adenosine deaminase (ADA) or common gamma chain (SCIDX1) defects, gene therapy can be applied. The single defective gene can be replaced as a treatment option.
Patients with ADA-SCID receiving gene therapy have significant long-lasting reconstitution of their T and B cells. These patients are also able to enhance their immune responses.
A novel and promising approach in gene therapy involves correcting defective genes within a patient’s hematopoietic stem cells (HSCs), either in vitro or directly in vivo. This method utilizes the advanced CRISPR/Cas9 gene-editing technology to replace mutated DNA sequences with normal ones.
Progress in bone marrow and hematopoietic stem cell (HSC) transplantation has enabled cell replacement therapy for certain immunodeficiencies. Currently, this remains the primary long-term treatment option for patients with severe combined immunodeficiency (SCID).
Administration of missing immunoglobulins in some disorders is known as protein replacement therapy. In various kinds of immunodeficiency, the injection of pooled human gammaglobulin, known as intravenous immunoglobulin (IVIG) protects against recurrent infections. To produce large quantities of purified proteins for treating deficiencies in enzymes, cytokines, and complement components, genes can be expressed in vitro using bacterial or eukaryotic expression systems. These proteins enable novel therapeutic approaches by replacing or increasing the levels of immunologically important proteins in patients.
You may also like:
- Immunodeficiency disorders can lead to the development of autoimmunity
- Defects in innate immune components result in deficiency and diseases
- Various factors cause secondary immunodeficiencies

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.