In this article, I briefly describe parasitic infections and how trypanosomes apply novel immune evasion strategies.
Parasitic Infections
Parasites are a vast category, from unicellular protozoan eukaryotes to macroscopic worms. The parasitic world is so diverse that it is difficult to generalize. Most eukaryotic protozoan parasites inhabit intracellular spaces in their human hosts for one life cycle stage or more. Conversely, multicellular eukaryotes like helminths are large in their adult stages and typically live and reproduce exclusively outside their host.
Protozoan Parasites- The Diverse Set of Unicellular Eukaryotes
Protozoan parasites cause the least treatable tropical diseases. These are unicellular eukaryotes, and many are motile and pathogenic. Many parasites, e.g., Giardia or Toxoplasma, are free-living and found in contaminated water. Other protozoan parasites move from their arthropod vector hosts (mosquitoes and flies) to their mammalian hosts. The movement happens when the insects suck blood during feeding. These entangled movements with multiple life stages make immune detection and elimination exacting.
Protozoan parasites do not follow a single, universal infection cycle, yet certain species of particular relevance to human health have been extensively studied. Notably, many of these parasites undergo transitions through various antigenic forms or inhabit different locations within the human host during their life cycle. This dynamic progression often allows them to stay one step ahead of the immune system’s defenses.
When the parasites stay in the bloodstream, gut, or interstitial fluid of their human host, humoral immunity provides the most effective defense. However, these stages are often brief or involve evasion mechanisms, leaving limited opportunities for clonal selection of lymphocytes or antibody binding. The parasites undergoing intracellular life cycle stages need cell-mediated immune reactions as a defense.
The Trypanosome- A Protozoan Parasite
African trypanosome is a protozoan parasite that causes a disease, African sleeping sickness. It’s a chronic disease transmitted to humans and cattle by the bite of the tsetse fly. In the bloodstream, this flagellated protozoan differentiates into a long, slender form that continues to divide every 4 to 6 hours. The trypanosomes multiply in the blood in an early systemic stage, which is the progressive point of the disease. In the systemic stage, trypanosomes multiply in the blood till the neurologic stage, at which the parasite infects cells of the central nervous system. The infection of the cells of the central nervous system leads to meningoencephalitis, which results in loss of consciousness.
The surface of the Trypanosoma parasite is covered with a variable surface glycoprotein (VSG). The organism escapes immunologic clearance by the extensive variation in these structures created by several unusual genetic processes. A single trypanosome carries a large repertoire of VSG genes, each encoding a different VSG primary sequence. However, the trypanosome expresses only a single VSG gene at a time. Trypanosoma brucei carries more than 1000 VSG genes in its genome. When a VSG gene gets activated, it is duplicated and transpositioned to a transcriptionally active expression site at the telomeric end of a specific chromosome (Figure 1).
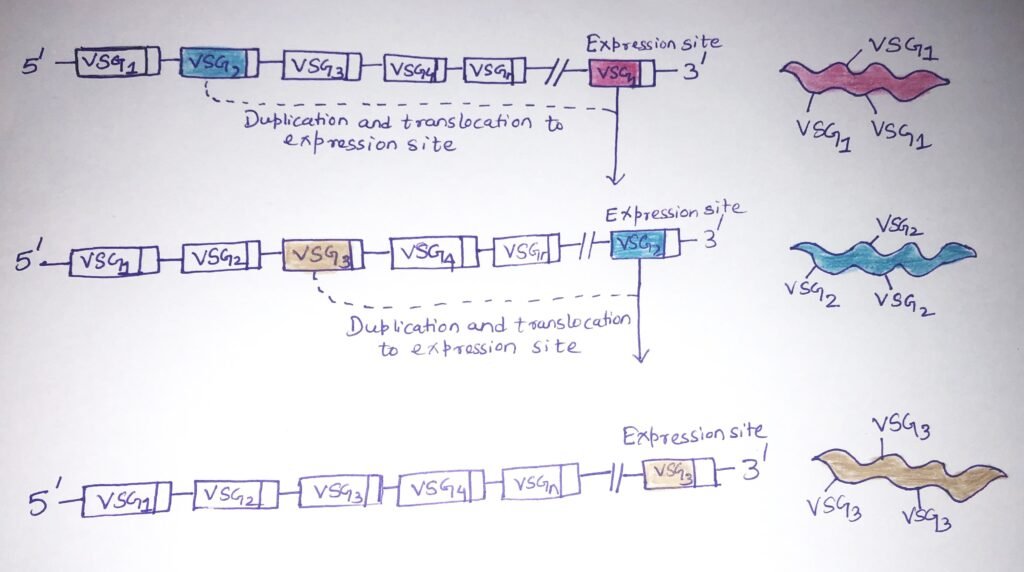
The activation of a new VSG gene displaces the previous gene from the telomeric expression site. Trypanosomes possess multiple transcriptionally active expression sites; thus, several VSG genes can potentially be expressed.
Parasites Evade the Immune Response
After infection, the number of parasites is increased, and the VSG covering the surface of the parasites elicits an effective humoral response. These antibodies discard most parasites from the bloodstream by complement-mediated lysis and opsonization with subsequent phagocytosis. The transposition of a different VSG gene into the expression site in about 1% of organisms leads them to bear an antigenically different VSG.
These parasites escape the initial antibody response. They begin to proliferate in the bloodstream. These parasites continue to populate the next wave of parasitemia in the host. The successive waves of parasitemia show a unique mechanism of antigenic shift through which the trypanosomes sequentially evade the immune response to their surface antigens. A single new variant arising in the course of a single infection escapes the humoral antibodies generated in response to the preceding variant. Thus, the recurrence of waves of parasitemia happens.
Helminths – The Metazoan Parasitic Worms Generate Weak Immune Responses
Metazoan parasites, commonly known as helminths, cause various diseases in both humans and animals. These adult helminths are large, multicellular organisms, often visible to the naked eye. The three primary categories of parasitic worms include nematodes (roundworms), cestodes (tapeworms), and trematodes (flukes). Most helminths enter their hosts via the intestinal tract, with their eggs capable of contaminating food, water, feces, and soil. However, certain species, such as schistosomes, can infect hosts directly through the skin.
Helminths are extracellular and thus more accessible to the immune system than protozoans. However, most infected individuals carry relatively few parasites at a time. Helminths, unlike protozoan parasites, do not multiply within their human hosts. This results in poor immune reactivity as fewer foreign epitopes are recognized. Phagocytic cells face difficulties in engulfing adult helminths. In this condition, the best approach would be expulsion rather than a typical humoral opsonization and digestion response.
IgE-mediated responses result in mast cell degranulation, which can help eject the worm from the body through the release of histamines and leukotrienes that induce muscle contractions and mucous production. A key characteristic of effective immune responses against metazoan parasites is their dependence on TH2-type mechanisms, which involve ILC2 activation, IL-4 production, TH2 cell stimulation, and a preference for IgE production over IgG.
Conclusion
Parasites are a very broad category, from unicellular protozoan eukaryotes to macroscopic worms. The diversity of the parasitic world makes it difficult to generalize about them. Protozoan parasites cause the least treatable tropical diseases. These are unicellular eukaryotes, and many are motile and pathogenic.
Humoral immunity is the most effective defense during the parasites’ stay in the bloodstream, gut, or interstitial fluid of their human host. The parasites undergoing intracellular life cycle stages need cell-mediated immune reactions as a defense.
African trypanosome is a protozoan parasite that causes a disease, African sleeping sickness. It’s a chronic disease transmitted to humans and cattle by the bite of the tsetse fly. The surface of the Trypanosoma parasite is covered with a variable surface glycoprotein (VSG). The organism escapes immunologic clearance by the extensive variation in these structures created by several unusual genetic processes.
Metazoan parasites, commonly known as helminths, cause various diseases in humans and animals. These adult helminths are large, multicellular organisms, often visible to the naked eye. Helminths are extracellular and thus more accessible to the immune system than protozoans. However, most infected individuals carry relatively few parasites at a time. Helminths, unlike protozoan parasites, do not multiply within their human hosts. This results in poor immune reactivity as fewer foreign epitopes are recognized.
You may also like:
- Role of innate and acquired immunity in controlling fungal infections
- Tumor Immune Escape Mechanisms and Apoptotic Resistance

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.



