In this article, I briefly describe the application of gene cloning in producing recombinant proteins.
Gene cloning
It is a molecular biology technique that makes identical copies of a particular piece of DNA, or gene. A target gene is introduced into a circular piece of DNA called a plasmid in a cloning experiment. The plasmid is then introduced into a bacteria via transformation (figure 1). Bacteria carrying the plasmid are selected using antibiotics. There are many applications of gene cloning. Cloning aids in producing proteins, vaccines, and antibiotics. Gene cloning helps to create pest-resistant plants in agriculture. In animal biotechnology, cloning is used to produce transgenic animals and has a role in gene therapy.
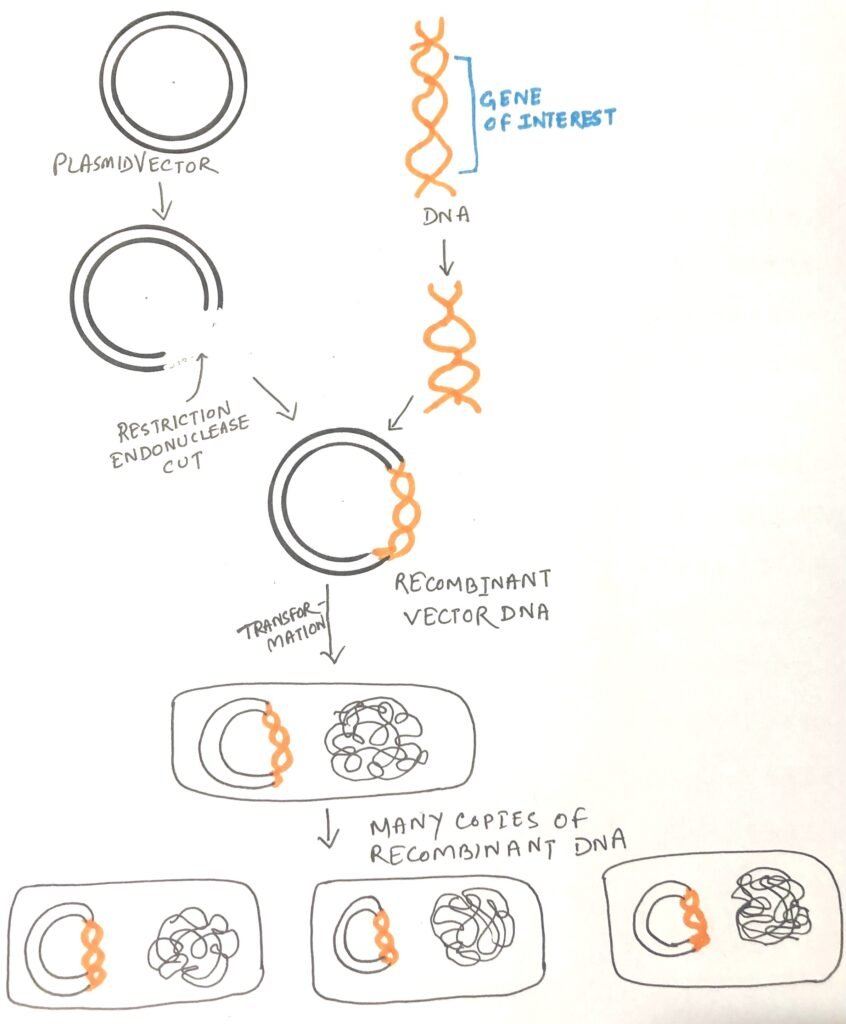
Production of recombinant protein
The proteins that are normally less produced include growth hormone, insulin in diabetes, interferon in some immune disorders, and blood clotting factor VIII in hemophilia, which are known to be missing or defective in various disorders. Previously before the advent of gene cloning and recombinant DNA technology, these molecules were purified from animal tissues or donated human blood. However, both sources have flaws, including slight functional differences in the non-human proteins and possible viral contamination. (e.g. HIV, CJD).
A gene responsible for producing a crucial protein in an animal or plant can be extracted from its source and inserted into a cloning vector. This vector can then be introduced into a bacterium. If the process is executed correctly, the gene will be expressed within the bacterial cell, leading to protein synthesis. This method allows for the production of large quantities of the protein. However, in practice obtaining recombinant protein is not as easy as theoretically it sounds. For this special types of cloning vectors are needed.
How foreign genes are expressed in E.coli
A foreign gene expresses itself in E.coli, depending on the signals surrounding the gene. These signals are short sequences of nucleotides, which provide instructions for the transcriptional and translational apparatus of the cell. The three most important signals for E.coli genes are:
1) The promoter, the transcription start site.
2) The terminator, the transcription stop site, and
3) The ribosome binding site, a short nucleotide sequence through which the ribosome is attached to the mRNA molecule.
An expression vector is a cloning vehicle designed to produce recombinant proteins by inserting a foreign gene under the control of specific E. coli expression signals.
For efficient gene expression, a strong promoter, an E. coli ribosome binding site, and a terminator are necessary. In most vectors, these expression signals form a cassette, as the foreign gene is inserted into a unique restriction site within the expression signal cluster. As a result, the foreign gene is positioned optimally to the expression signals when it is ligated into the cassette.
The foreign gene must be inserted into this restriction site in a manner that fuses the two reading frames, creating a hybrid gene that begins with the E. coli segment and seamlessly continues into the codons of the foreign gene. As a result, the expressed gene product is a hybrid protein, composed of a short peptide encoded by the E. coli reading frame joined to the amino-terminus of the foreign protein.
The difficulties in producing recombinant protein in E.coli
Many problems are associated with protein production from foreign genes cloned in E.coli. These obstacles may be due to the sequence of the foreign gene, and due to the limitations of E.coli as a host for recombinant protein synthesis.
The foreign gene might contain introns, which causes a problem as E.coli genes don’t contain introns. The bacterium thus doesn’t possess the required machinery for removing introns from transcripts. Sequences present in the foreign gene can act as termination signals in E.coli. The codon usage of the gene may not be ideal for translation in E.coli. The bacterium might not process the recombinant protein correctly. The improper folding of E. coli often leads to improper tertiary structure making the protein insoluble and causing it to form an inclusion body inside the bacterium. Thus, converting the protein into its properly folded form is nearly impossible, and the protein remains inactive. E.coli might degrade the recombinant protein.
Challenges in achieving high yields of active recombinant proteins from genes cloned in E. coli have prompted the development of expression systems in higher organisms. Yeasts and fungi can be cultured as easily as bacteria capable of expressing cloned genes from higher organisms.
Recombinant protein from yeast
The yeast Saccharomyces cerevisiae is the most widely used microbial eukaryote for recombinant protein production. Cloned genes are typically placed under the control of the GAL promoter, which is normally located upstream of the gene encoding galactose epimerase, an enzyme involved in galactose metabolism. The GAL promoter is activated by galactose, thereby regulating the expression of the cloned gene.
While S. cerevisiae can achieve relatively high yields of recombinant proteins, it cannot glycosylate animal proteins correctly and lacks an efficient system for secreting proteins into the growth medium. Without secretion, recombinant proteins remain within the cell, making them more difficult to purify. Despite these limitations, S. cerevisiae remains the most commonly used microbial eukaryote for recombinant protein production.
Filamentous fungi produce recombinant proteins
The advantages of fungi in recombinant protein production include their efficient glycosylation capabilities and ability to secrete proteins directly into the growth medium. The wood rot fungus Trichoderma reesei, e.g., naturally secretes cellulolytic enzymes that break down the wood it inhabits. These fungi produce recombinant proteins in a form that facilitates purification. Another commonly used fungus for recombinant protein production is Aspergillus nidulans. Expression vectors for A. nidulans typically use the glucoamylase promoter, which is activated by starch and suppressed by xylose.
Recombinant protein production from animal cells
For proteins with complex and essential glycosylation patterns, animal cells may be the only viable hosts capable of synthesizing active proteins. However, a challenge with some animal cell lines is their requirement for a solid surface to grow on, complicating the design of culture vessels. Additionally, animal cells have lower growth rates and maximum cell densities compared to microorganisms. It limits the yield of recombinant proteins. Two promoters commonly used in mammalian cells. The first one is the heat shock promoter from the human hsp-70 gene, activated at temperatures above 40°C. The second promoter is from the mouse metallothionein gene, which is triggered by, the addition of zinc salts to the culture medium.
Conclusion
Gene cloning has many applications in the production of proteins, vaccines, and antibiotics. Gene cloning helps to produce pest-resistant plants in agriculture. In animal biotechnology, cloning is used to produce transgenic animals and has a role in gene therapy.
A gene responsible for producing a crucial protein in an animal or plant can be extracted from its source and inserted into a cloning vector. This vector can then be introduced into a bacterium. If the process is executed correctly, the gene will be expressed within the bacterial cell, leading to protein synthesis.
A foreign gene expresses itself in E.coli by depending on the signals surrounding the gene. The promoter, the terminator, and the ribosome binding site are the three most important signals for E.coli genes. The protein production from foreign genes cloned in E. coli faces certain challenges. These issues may arise from the sequence of the foreign gene itself, as well as the inherent limitations of E. coli as a host for recombinant protein synthesis.
The yeast Saccharomyces cerevisiae is the most widely used microbial eukaryote for recombinant protein production. Fungi offer advantages in recombinant protein production due to their effective glycosylation abilities and capacity to secrete proteins into the growth medium.
For proteins requiring complex and critical glycosylation, animal cells might be the only suitable hosts for producing active proteins. However, a challenge with certain animal cell lines is their need for a solid surface to grow on, which adds complexity to the design of culture vessels.
You may also like:

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.



