In this article, I briefly describe the application of gene cloning in medicine.
Gene Cloning
Gene cloning begins with isolating and inserting a specific gene into a vector. The vector is then introduced into a host organism. It makes multiple, identical copies of a particular gene or DNA segment. The host organism then replicates, producing copies of the inserted gene and DNA. This technique is fundamental in genetic research, biotechnology, and medicine. It allows scientists to study genes in detail, produce proteins, and develop genetic therapies. Medicine will continue to be a major beneficiary of the gene cloning revolution.
Production of Recombinant Insulin
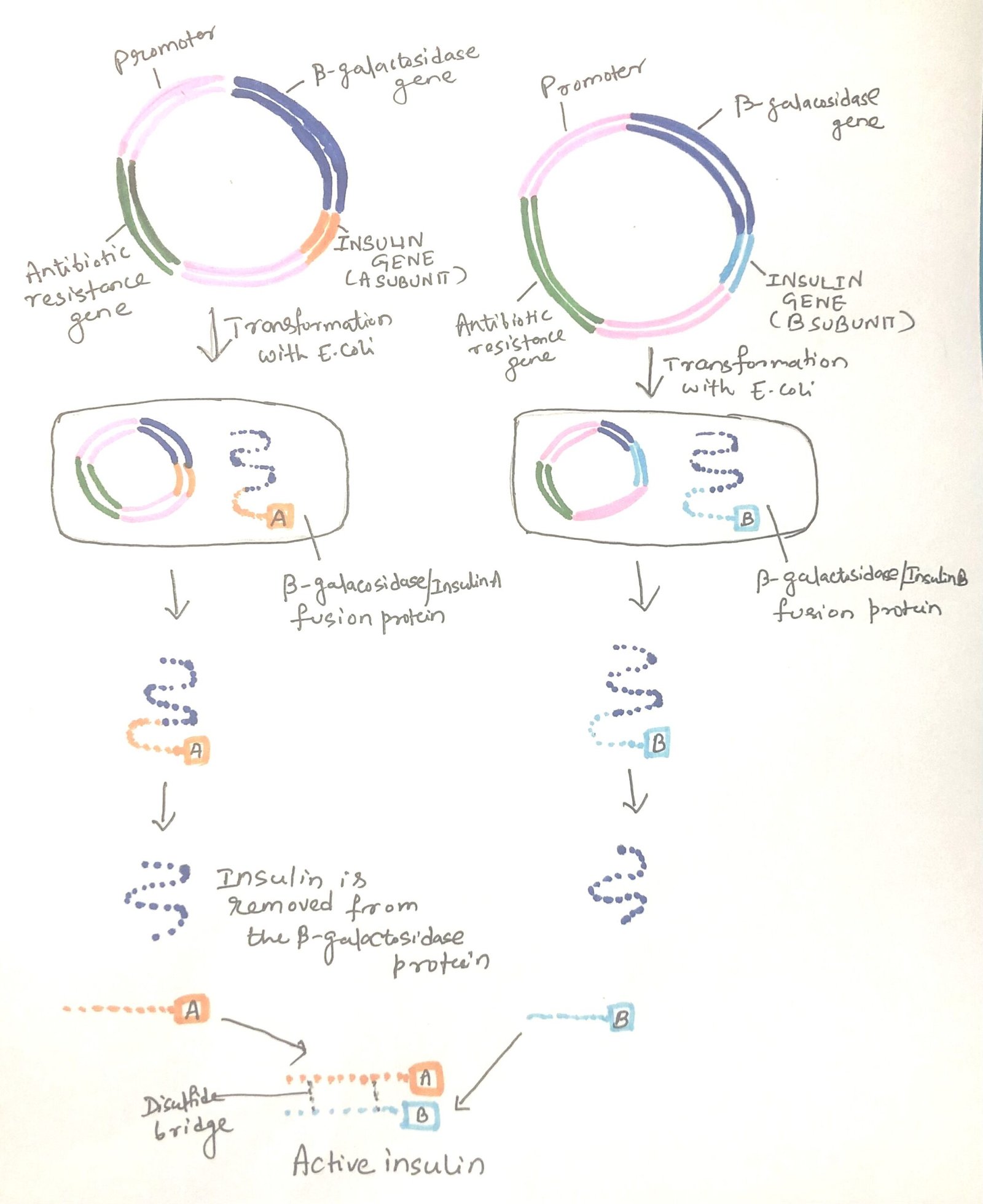
Insulin, produced by the β-cells in the Islets of Langerhans within the pancreas, regulates blood glucose levels. A lack of insulin leads to diabetes mellitus, a serious and potentially life-threatening condition if untreated. Insulin is a relatively small protein, made up of two polypeptide chains. In humans, insulin is initially produced as a precursor called pre-pro-insulin, which includes the A and B chains connected by a third chain, C, along with a leader sequence at the beginning. After translation, the leader sequence is removed, and the C chain is cut out, leaving the A and B chains connected by two disulfide bonds (Figure 1).

To synthesize and express artificial insulin genes, researchers constructed two recombinant plasmids. One plasmid contains the artificial gene for the A chain and another for the B chain. In both cases, these genes were linked to a lacZ’ reading frame in a pBR322-type vector, placing them under the control of the strong lac promoter. This setup allowed the insulin genes to be expressed as fusion proteins, with the first few amino acids of β-galactosidase followed by the A or B polypeptides (Figure 1).
A methionine residue was inserted between the β-galactosidase and insulin segments, enabling the insulin polypeptides to be separated from the β-galactosidase by cyanogen bromide treatment. The isolated A and B chains were then joined by disulfide bond formation in vitro. A later enhancement involved synthesizing the entire pro-insulin reading frame, which includes the B chain, C chain, and A chain. This pro-hormone can spontaneously fold into the correct structure with disulfide bonds, and the C chain can be removed through proteolytic cleavage.
Working of a Fusion Protein
Fusion proteins are engineered molecules created by joining two or more genes that originally coded for separate proteins. This genetic fusion results in a single protein with combined functions or properties derived from each of the original proteins.
The genes coding for the desired proteins are fused at the DNA level. This can involve attaching the gene of interest to a tag (like GFP for visualization) or another functional protein (like an enzyme or antibody fragment).
A short DNA sequence coding for a flexible peptide linker may be inserted between the fused genes. This linker ensures that the individual protein domains can function properly without interfering with each other.
The fused gene is inserted into an expression vector (e.g., a plasmid), which is then introduced into a host cell (like bacteria, yeast, or mammalian cells). The host cell machinery transcribes the fused gene into mRNA and then translates it into a single polypeptide chain, the fusion protein.
Production of Other Recombinant Human Proteins and Recombinant Vaccines
Human cloning of the genes in bacteria and eukaryotic cells has produced numerous growth factors. These growth factors include somatostatin, somatotropin, factor VIII, Interferon-α, Interferon-β, Interferon-γ, interleukins, etc. These are used in the treatment of various diseases and have potential applications in cancer therapy. Since these proteins are naturally produced in the body in tiny amounts, recombinant technology is the only feasible way to obtain them in the quantities required for clinical use.
Vaccines are Produced as Recombinant Proteins
The application of gene cloning in vaccine development is based on the finding that virus-specific antibodies can be generated not only in response to the virus but also to isolated viral components. This is particularly evident with purified proteins from the virus coat. If the genes that code for these antigenic proteins can be identified and inserted into an expression vector, the same methods used to synthesize animal proteins could be applied to produce recombinant proteins as potential vaccines.
These vaccines would offer the advantage of being free from intact virus particles and could be produced in large quantities. However, this approach has not been entirely successful. The recombinant coat proteins often do not fully replicate the antigenic properties of the intact virus. Nevertheless, there has been success with the hepatitis B virus, where its coat protein was synthesized in Saccharomyces cerevisiae using a vector based on the 2μm plasmid. The protein was produced in relatively high quantities and, when injected into monkeys, protected against the hepatitis B virus. This recombinant vaccine has been approved for human use.
Live Recombinant Vaccine
Recombinant vaccinia viruses have the potential to be used as live vaccines against other diseases. If a gene coding for a viral coat protein is inserted into the vaccinia genome under the control of a vaccinia promoter, the gene will be expressed. Following injection into the bloodstream, replication of the recombinant virus will produce new vaccinia particles and significant quantities of the major surface antigen.
Gene Cloning Helps to Identify Genes Responsible for Human Diseases
A genetic disease results from a defect in a particular gene. Individuals with this defective gene have an increased likelihood of developing the disease at some point in their lives. Identifying the gene can reveal the biochemical basis of the disease, which may lead to the development of targeted therapies. Detecting the specific mutation in a defective gene can also enable the creation of screening programs. It helps to identify individuals who carry the mutant gene or have not yet shown symptoms of the disease. Additionally, identifying the gene is essential for the possibility of gene therapy.
Mapping the Breast Cancer Gene BRCA1
Accurate gene mapping is essential for isolating a gene. Linkage analysis involves comparing the inheritance pattern of the target gene with the inheritance patterns of genetic loci that have already been mapped. If two loci are inherited together, they must be located very close to each other on the same chromosome. In the case of breast cancer, a significant breakthrough occurred in 1990 when a team at the University of California at Berkeley used RFLP linkage analysis.
Their study revealed that in families with a high incidence of breast cancer, many affected women shared the same version of an RFLP called D17S74. This RFLP had previously been mapped to the long arm of chromosome 17, indicating that the BRCA1 gene must also be located on this chromosome’s long arm. To more precisely pinpoint BRCA1, researchers examined the chromosomal region containing BRCA1 for tandem repeat sequences. These sequences are similar to those used in genetic fingerprinting but with tiny repeat units.
These repeat sequences are valuable in linkage analysis. This is because the number of repeats at a locus can vary greatly, likely due to occasional replication errors that add extra units. Linkage is determined by comparing the inheritance patterns of a locus with a specific repeat length and the gene of interest. By using the repeat loci identified on chromosome 17, this approach narrowed down the BRCA1-containing region from 20 Mb to just 600 kb.
Application of Cloning in Medicine- Gene Therapy
Gene therapy is used to correct defective genes causing diseases. It involves the insertion, modification, or removal of genes within a person’s cells and tissues to treat diseases. It is considered the most valuable application of cloning in medicine.
Somatic Cell Therapy
Gene therapy often involves manipulating regular cells, typically by removing them from the organism. Then, transfecting them with new genes and reintroducing them into the body. This technique shows great promise for treating inherited blood disorders. Here, genes are introduced into stem cells from the bone marrow, which then develop into all the various blood cell types. Additionally, somatic cell therapy holds potential for treating lung diseases like cystic fibrosis. In this approach, DNA is introduced into the respiratory tracts of rats using an inhaler, where it is absorbed by the epithelial cells in the lungs. However, gene expression typically lasts only a few weeks.
Germline Therapy
Germline gene therapy is a form of gene therapy that involves modifying the genetic material of reproductive cells, such as eggs and sperm, or early embryos. A fertilized egg is injected with a correct version of the relevant gene and then reimplanted into the mother. If successful, this gene is incorporated into and expressed by all cells of the resulting individual. This approach could be used either to prevent a genetic disease or to introduce a beneficial genetic variation. However, there have been no trials of human germline gene therapy to date.
The introduced therapeutic DNA must remain functional, which causes gene therapy, a permanent cure. The cells carrying this DNA must be durable and stable. Challenges in integrating therapeutic DNA into the genome, combined with the rapid cell division in many tissues, hinder the achievement of long-term benefits. As a result, patients may need to undergo multiple rounds of gene therapy.
Conclusion
Gene cloning is fundamental in genetic research, biotechnology, and medicine. It allows scientists to study genes in detail, produce proteins, and develop genetic therapies. Medicine will continue to be a major beneficiary of the gene cloning revolution.
Researchers constructed two recombinant plasmids to synthesize and express artificial insulin genes. Fusion proteins are engineered molecules created by joining two or more genes that originally coded for separate proteins. This genetic fusion results in a single protein with combined functions or properties derived from each of the original proteins. Insulin genes are expressed as fusion proteins.
Recombinant vaccinia viruses have the potential to be used as live vaccines against other diseases. If a gene coding for a viral coat protein is inserted into the vaccinia genome under the control of a vaccinia promoter, the gene will be expressed.
A genetic disease results from a defect in a particular gene. Individuals with this defective gene have an increased likelihood of developing the disease at some point in their lives. Identifying the gene can reveal the biochemical basis of the disease, which may lead to the development of targeted therapies.
Gene therapy often involves manipulating regular cells, typically by removing them from the organism. Then, transfecting them with new genes and reintroducing them into the body. This technique shows great promise for treating inherited blood disorders. Germline gene therapy is a form of gene therapy that involves modifying the genetic material of reproductive cells.
You may also like:

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.