In this article, I briefly describe animal viruses and their use as cloning vectors.
Cloning vectors
A small piece of DNA into which a foreign DNA fragment is inserted is known as a cloning vector. The cloning vector may be the plasmid from a bacterium, a higher organism’s cell, or DNA taken from a virus. The vector contains restriction sites that help conveniently insert and remove a DNA fragment. The cloning vector and the foreign DNA are treated with a restriction enzyme that creates the same overhang, then ligating the fragments together. Genetically engineered plasmids and bacteriophages (such as phage λ) are perhaps most commonly used. Other types of cloning vectors are also used, which include bacterial artificial chromosomes (BACs) and yeast artificial chromosomes (YACs).
Animal viruses and their use as cloning vector
To construct a vector based on animal viruses, certain things need to be carefully observed. The vector genome must be easily manipulated. It must contain convenient restriction sites for the restriction enzymes. In any eukaryotic cloning vector, efficient expression of the inserted gene is required. Like bacteriophage vectors, many animal vectors also face rigid packaging constraints due to the large size of eukaryotic genes. Viruses can carry such large-sized eukaryotic genes. To avoid packaging constraints, it’s highly desirable to create vectors, which replicate episomally. Thus, they do not need to pass through an infectious virion. The ideal vector would be capable of delivering the cloned gene into many specialized cell types derived from various species.
SV40 Virus
SV40 or simian virus 40 was the first eukaryotic DNA virus, for which a complete nucleotide sequence and a thorough understanding of transcription were available. It is a polyomavirus, which is found in both monkeys and humans. The genome of SV40 contains very little non-essential DNA. Thus, the foreign gene is inserted in place of essential viral genes and the recombinant genome is propagated in the presence of a helper virus.
This virus is capable of infecting several mammalian species, following a lytic cycle in some hosts and a lysogenic cycle in others. The viral genome is 5.2kb in size and contains two sets of genes, the early genes and the late genes. The early genes are expressed early in the infection cycle and coding for proteins involved in viral DNA replication. The late genes code for viral capsid proteins (figure 1). However, the small size of the viral genome ( 5.24 kb), highly constrains the propagation of recombinant DNA molecules and restricts the packaging limit. Therefore, these can not be used for the analysis of most eukaryotic genes.

Adenoviruses
These are a group of viruses that enable the cloning of larger DNA fragments. Due to their larger genome size, adenoviruses are more difficult to handle. Adenoviruses are non-enveloped viruses with an icosahedral nucleocapsid containing a double-stranded DNA genome (figure 2) of 30-40 kb. The core and the capsid are the main components of adenoviruses.
The capsid consists of the homotrimeric hexon capsomers, which form the 20 triangular faces of the icosahedron. Each of the 12 vertices on the adenovirus surface is made up of a penton capsomere. The penton capsomere is composed of a penton base and a fiber protein. The virus attaches to its primary receptor with the help of a knob, which is formed by the fiber protein. There is the presence of various hexon-associated proteins with capsid stabilizing functions along with hexon and penton capsomeres. The double-stranded DNA genome is located in the virion core with DNA-associated proteins and terminal proteins that serve as a primer for DNA replication.
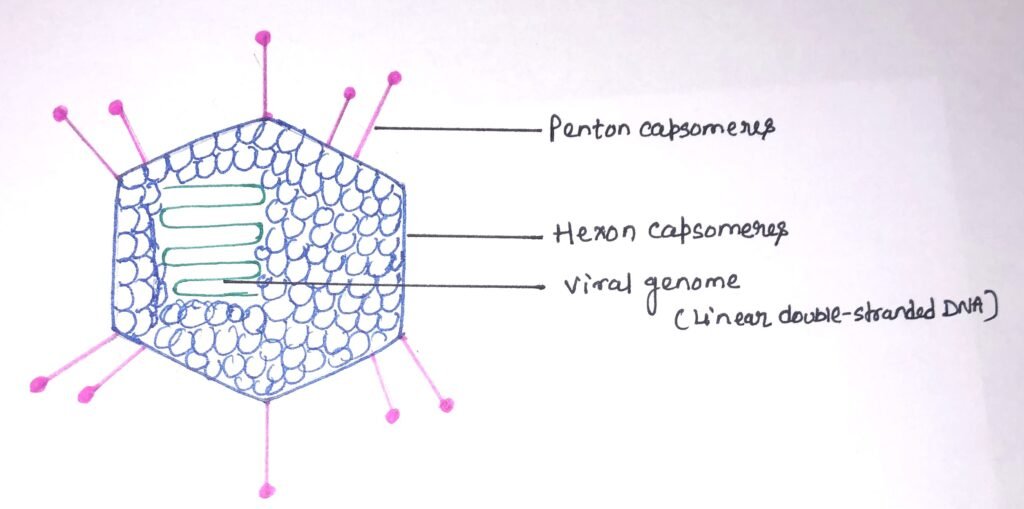
Due to their ability to accommodate large exogenous gene fragments, adenoviruses are used as potential vaccine candidates against pathogenic diseases. Recombinant adenoviruses are effective tools for high-efficiency gene transfer and gene therapy due to their ease of production, high transduction efficiency, and low immunogenicity. Scientists are continuously modifying the adenovirus genome to enable it to accommodate larger transgenes and to lower its immunogenicity.
Papillomaviruses
For transferring genes into living mammalian cells, the papillomaviruses are used as a valuable tool. They also have a relatively high capacity for inserted DNA. Cloning vectors have been developed from several animal viruses like SV40 and polyomavirus, herpesviruses, adenoviruses, poxviruses, and retroviruses. However, infection from these viruses culminates in cell death. Thus, it is almost impossible to establish permanent cell lines for long-term biochemical and genetic manipulation. To combat with these problems, a new class of eukaryotic cloning vectors has been developed from BPV-l (Bovine papillomavirus-1) DNA.
Papillomavirus-transformed cells don’t contain integrated viral DNA rather they contain between 50 and 300 copies of unintegrated, circular viral DNA although some proportion of these viral genomes exists as concatamers and/or catenates. Bovine papillomavirus type 1 is the most suitable cloning vector among other types. It causes warts on cattle. The BPV has an unusual infection cycle in mouse cells and appears in the form of a multi-copy plasmid with about 100 molecules present per cell. It doesn’t cause the death of the mouse cell. Shuttle vectors consisting of BPV and pBR322 sequences, possess the capability of replicating in both mouse and bacterial cells and therefore have a great value in animal cell biotechnology.
The Bovine papillomavirus-1
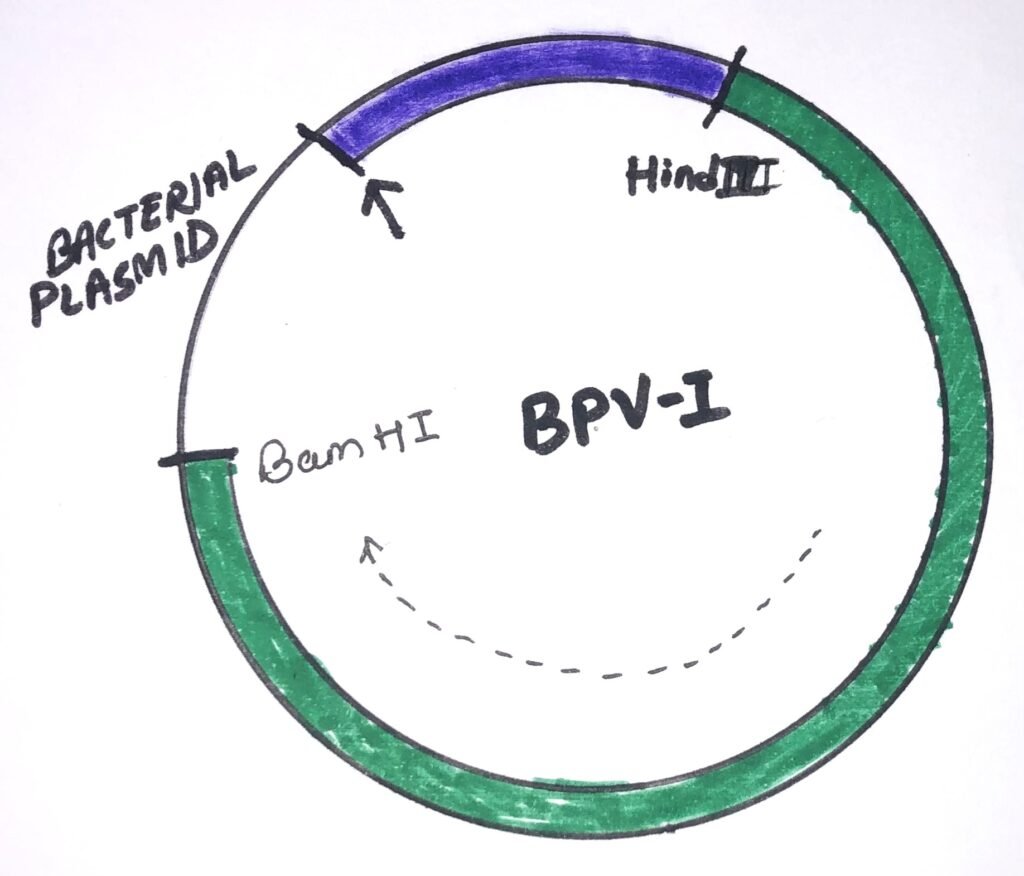
An idealized BPV-I cloning vector (figure 3) shows a circle representing a circular, double-stranded DNA molecule. The green segment represents the 5500-bp HindIII to BamHI restriction fragment of BPV-l DNA, which is enough to transform mouse cells. The dashed arrow shows the direction of transcription of this fragment. The purple zone is a segment of DNA, which stimulates mouse cell transformation by the BPV-l DNA fragment. Such segments include the remaining 2500 bp of the viral genome and any of several DNA fragments from cellular genomes. The bacterial plasmid segment contains sequences that allow selection and amplification of the plasmid in bacteria. The small arrow at a position indicates the insertion of an additional DNA segment can be possible. The inserted DNA segment into the vector must not disturb an essential gene or regulatory signal of the viral DNA.
Retroviruses
This virus has a single-stranded RNA genome and inserts a DNA copy of its RNA genome into the DNA of a host cell that it invades. Thus, it changes the genome of the host cell. When it infects a cell, the RNA genome is reverse-transcribed into DNA, which subsequently integrates into the host genome. Genomic transcription of the viral genes generates viral genomic RNA or RNA encoding viral proteins. They can alter the host genome and they can be converted into replication defective vectors easily. This makes them the most promising vector system of all.
The structure of Retrovirus
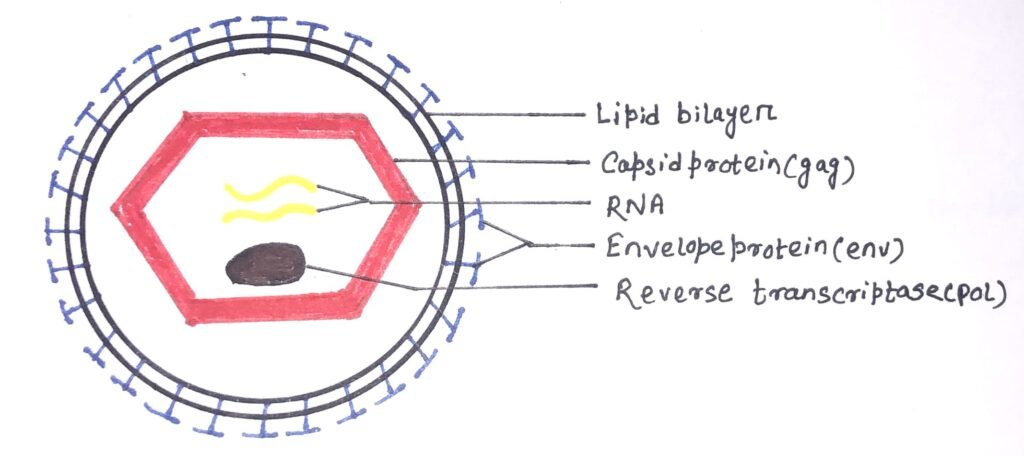
A retrovirus consists of two concentric outer circles of the lipid bilayer that contain the envelope protein complex embedded in it. The main viral components are the viral envelope, RNA, and proteins. The capsid proteins are hexagonal (figure 4). The retroviral envelope is composed of lipids and glycoproteins encoded by env genes. The envelope protects the virus from the external environment and also helps it to exit and enter the host cell.
It also directs the viral entry into the host cell by fusing with the host cell membrane. The viral RNA consists of a dimer with a 5′ cap and a 3′ poly-A tail. At the terminal, It has non-coding regions, which help in replication. The internal regions encode virion proteins for gene expression. The virus consists of four types of proteins, i.e., gag proteins, pol proteins, protease, and env proteins. The three types of retroviruses affecting humans are HIV, the human T-cell lymphotropic virus, and endogenous retrovirus.
During the process of reverse transcription, sequences from the termini of viral RNA are duplicated to generate long terminal repeats(LTRs). These long terminal repeats include both the promoter and the polyadenylation signal for the transcription of viral mRNAs. The long terminal repeats also determine the specificity of proviral DNA integration. Retroviruses can integrate at many sites within the cellular genome. However, integrative recombination always occurs at particular sites at the ends of the LTRs. The sequences appropriately inserted between the two LTRs will be integrated intact. However, it differs sharply from the integration of papovavirus or adenovirus DNA, during which extensive rearrangements of the integrated viral sequences take place. Retroviruses have an advantage over others as they are natural transducing viruses.
Baculoviruses
These are a group of viruses, which infect insects. Baculoviruses infect a large range of insects mainly deriving from the orders Diptera (flies), Hymenoptera (wasps, bees, and ants), and Lepidoptera (butterflies and moths). These are very small, rod-shaped structures. These viruses comprise a protein coat, which encodes components for further viral reproduction. Baculoviruses contain a genome of double-stranded DNA of around 130kb. The DNA is infectious. The genome is capable of producing very large quantities of transgene protein, but until recently the system was limited to insect cells. Recent developments in research have shown the potential of the baculovirus vector system for use in mammalian cells.
Baculovirus contains a small amount of DNA, which enables large amounts of proteins to be obtained from genes cloned in insect cells. One of the major proteins encoded by the virus genome is polyhedrin, which accumulates in very large quantities in the nuclei of infected cells. The large accumulation is due to the presence of an extremely active promoter in the gene. The same promoter can be used to drive the overexpression of a foreign gene engineered into the baculovirus genome, which leads to the production of large quantities of protein in infected insect cells in culture. This method is being used increasingly for large-scale cultures of proteins of animal origin.
Production of Baculovirus vectors
The homologous recombination between a shuttle vector and a wild-type genome, using the viral polyhedrin gene leads to the production of baculovirus vectors. Shuttle vectors contain the transgene of interest along with a selectable marker gene. The insertion of transgene causes the inactivation of the polyhedrin gene, which gives rise to plaques that can be differentiated from those of wild-type viruses. In infected tissue culture cells, the expression of polyhedrin protein can continue for days following initial infection and often amounts to 50% of the total cell protein. The polyhedrin protein is not essential for viral replication in tissue culture. The virus has a rod-shaped capsid and has a large capacity for packaging foreign DNA. Modifications to the baculovirus vector have made the transgene expression possible in a variety of mammalian cell lines, further expanding the potential of the vector system.
Conclusion
The cloning vector may be the plasmid from a bacterium, a higher organism’s cell, or DNA taken from a virus. The vector contains restriction sites that help conveniently insert and remove a DNA fragment. Animal viruses are capable of carrying larger eukaryotic genes.
SV40 or simian virus 40 was the first eukaryotic DNA virus, for which a complete nucleotide sequence and a thorough understanding of transcription were available. It is a polyomavirus, which is found in both monkeys and humans. This virus is capable of infecting several mammalian species, following a lytic cycle in some hosts and a lysogenic cycle in others.
Adenoviruses are a group of viruses which enable cloning of larger DNA fragments. Due to their larger genome size, adenoviruses are more difficult to handle. Due to their ability to accommodate large exogenous gene fragments, adenoviruses are used as potential vaccine candidates against pathogenic diseases.
The papillomaviruses are used for transferring genes into living mammalian cells. Bovine papillomavirus type 1 is the most suitable cloning vector among other types.
Retrovirus has a single-stranded RNA genome and inserts a DNA copy of its RNA genome into the DNA of a host cell that it invades. Retroviruses can alter the host genome and they can be converted into replication defective vectors easily. This makes them the most promising vector system of all.
Baculoviruses infect insects. The virus has a rod-shaped capsid and has a large capacity for packaging foreign DNA. Modifications to the baculovirus vector have made the transgene expression possible in a variety of mammalian cell lines, further expanding the potential of the vector system.
You may also like:

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around twelve years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.
