In this article, I briefly describe how viruses cause different types of cancer. This disease has long been viewed primarily as a genetic disease driven by mutations within cells. However, growing scientific evidence has revealed that certain viruses play a crucial role in initiating and promoting malignant transformation. These oncogenic viruses interfere with normal cellular regulatory mechanisms through genome integration, persistent expression of viral proteins, or chronic inflammation. Understanding the molecular basis of viral oncogenesis has not only deepened our knowledge of cancer biology but has also opened new avenues for prevention and therapeutic intervention.
Fundamental Features of Cancer Development
Cancer comprises a diverse group of diseases characterized by uncontrolled cell growth and abnormal tissue development. More than one hundred clinically distinct types of cancer have been identified, each presenting unique symptoms and requiring specific therapeutic approaches. Despite this diversity, most cancers can be broadly classified into four major categories: leukemias, lymphomas, sarcomas, and carcinomas.
Leukemias are cancers that arise from the bone marrow and involve excessive production of abnormal white blood cells. Lymphomas develop when abnormal lymphocytes accumulate within lymph nodes, the spleen, or other lymphoid tissues. Sarcomas are solid tumors that originate from mesodermal tissues, including bone, cartilage, muscle, connective tissue, and adipose tissue. In contrast, carcinomas arise from epithelial tissues and represent the most common type of cancer. Epithelial tissues form the protective coverings of the body’s internal and external surfaces and include the skin, glandular tissues, nervous tissue derivatives, breast tissue, and the lining of the respiratory, digestive, urinary, and reproductive systems.
Cancer Characteristics and Early Evidence of Viral Involvement
Cancer is commonly defined by three fundamental biological features. These can be distinguished as hyperplasia, anaplasia, and metastasis. Hyperplasia refers to uncontrolled and excessive cell division, resulting in abnormal tissue growth. Anaplasia describes the loss of normal cellular structure and differentiation, causing cancer cells to appear structurally irregular and poorly specialized. Metastasis represents the ability of malignant cells to detach from the primary tumor, travel through the body, and establish secondary tumors at distant sites.
The possibility that viruses contribute to cancer development has intrigued scientists for many decades. Early experimental studies suggested a relationship between viral infections and certain cancers. In the early twentieth century, researchers demonstrated that some leukemias and sarcomas in chickens were caused by viral agents. Initially, these discoveries were considered limited to animals and were not believed to be relevant to human cancers, particularly because cancer in humans did not appear to spread through infectious transmission. However, extensive research in recent decades has provided strong evidence that several viruses can induce cancer in animals. These findings have renewed scientific interest in the role of viruses as potential contributors to human cancer development.
Oncogenic Viruses Induce Tumor Development
Both RNA and DNA viruses are capable of causing cancer in animals. When these viruses infect susceptible cells, they alter normal cellular regulation, leading to uncontrolled growth and tumor formation. Viruses that have the ability to initiate such tumor development are known as oncogenic viruses.
Among RNA-containing viruses, only certain members of the Retroviridae are known to induce cancer in animals. These viruses are unique because, although their genetic material is RNA, they replicate through a DNA intermediate. Other RNA viruses, which replicate solely via RNA intermediates, have not been shown to cause cancer. In contrast, several DNA virus families, including Herpesviridae, Adenoviridae, and Papovaviridae, contain members capable of inducing tumors in animals.
Viral Genome Integration and Provirus Formation
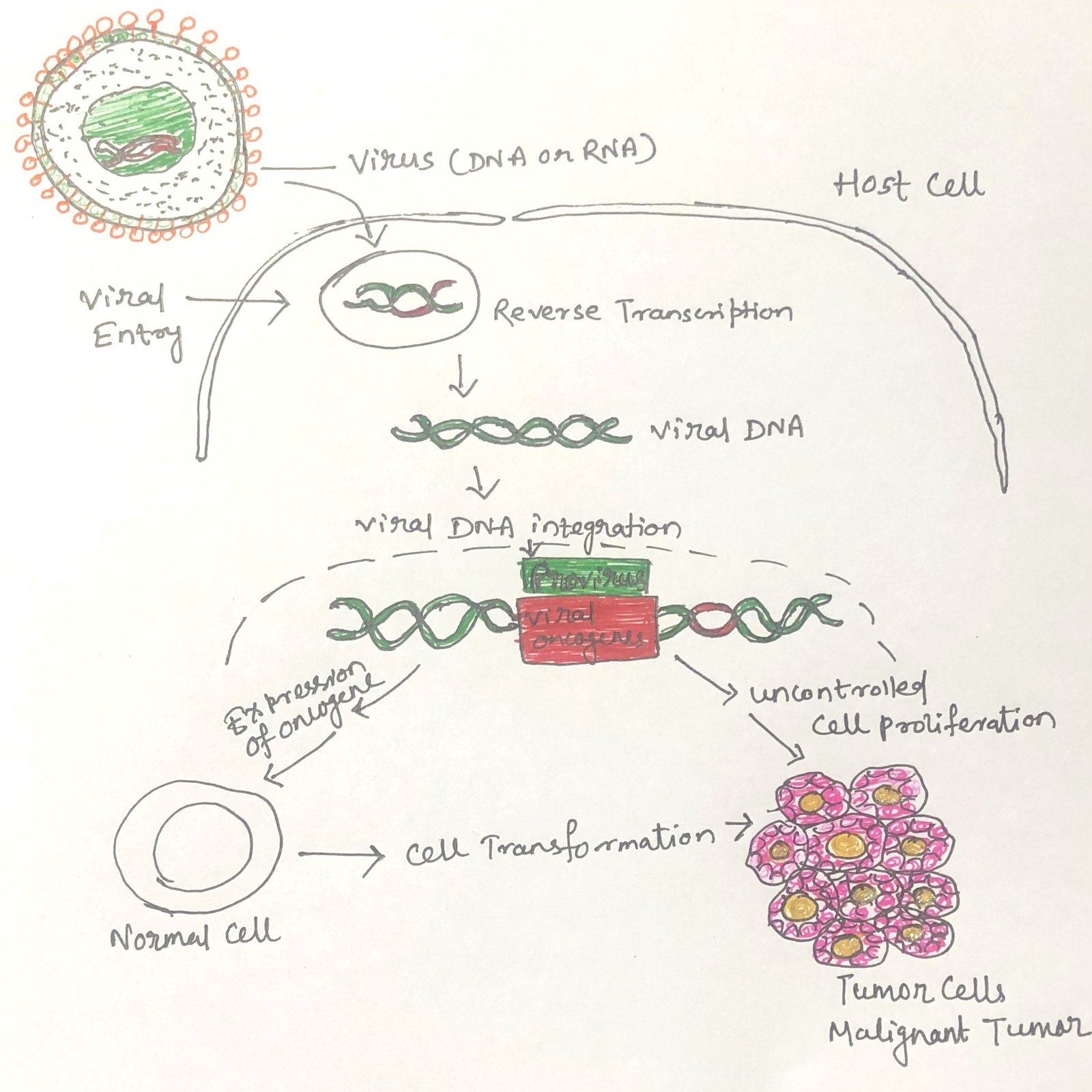
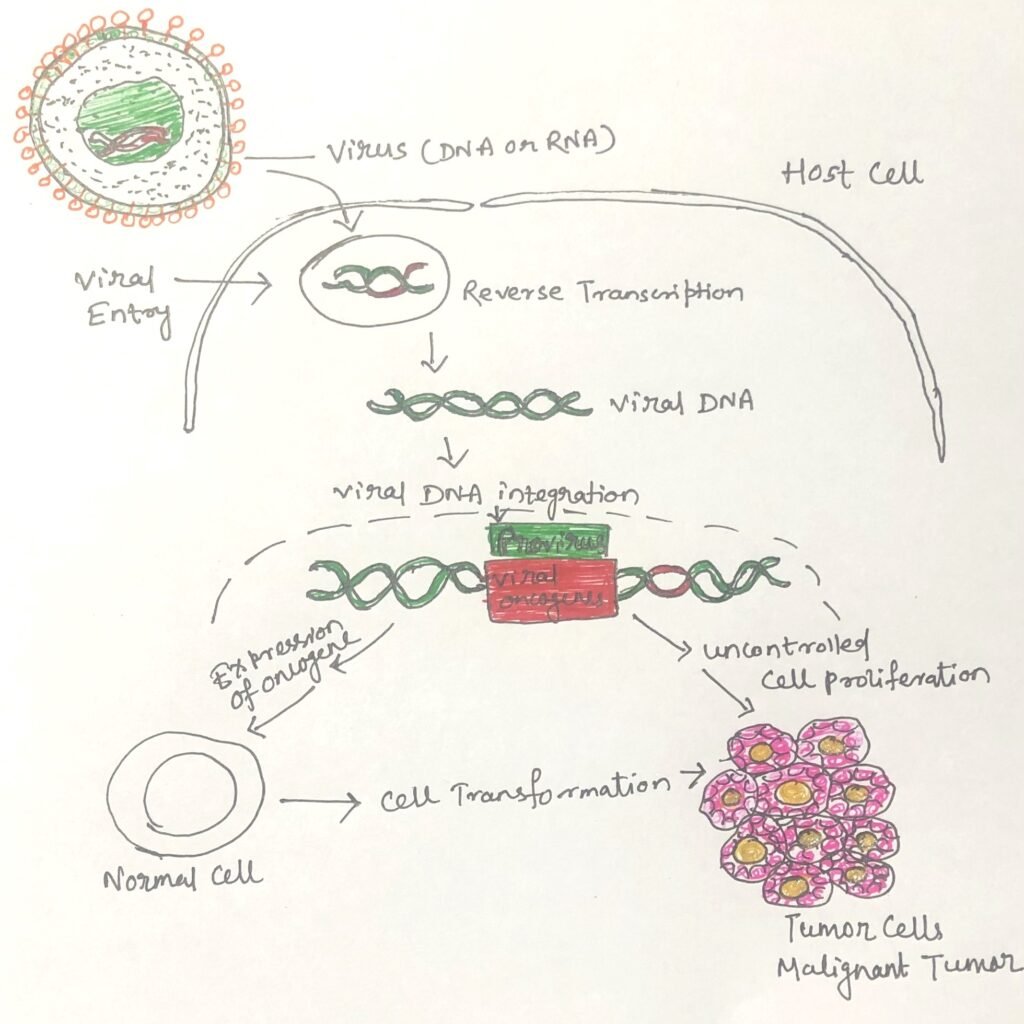
A defining feature of cancer-causing viruses is their ability to direct the production of viral DNA within the host cell nucleus. This DNA encodes proteins that are not structural components of the virus particle but instead contribute to cellular transformation. In all oncogenic viruses, the viral genome becomes integrated into, or closely associated with, the host cell’s DNA. Importantly, the infected host cell typically does not undergo lysis; instead, the viral genome persists within the cell in a manner comparable to lysogeny observed in bacteria infected by temperate bacteriophages.
In the case of RNA tumor viruses, the viral RNA acts as a template for synthesizing complementary DNA. This process is catalyzed by the enzyme reverse transcriptase, which forms a DNA–RNA hybrid molecule. Subsequently, a standard DNA polymerase synthesizes a complementary DNA strand, producing a double-stranded DNA molecule known as a provirus (Figure 1). Once formed, the provirus integrates into the host genome, where it can disrupt normal cellular regulation and promote transformation, ultimately leading to tumor development.
Variations in Oncogenic Potential Among Tumor Viruses
Cancer-causing viruses do not all possess the same capacity to induce tumors. Certain RNA tumor viruses, some members of the herpesvirus group, and a single papovavirus are capable of producing cancers under natural conditions, outside controlled laboratory settings. In contrast, other RNA tumor viruses, adenoviruses, and the majority of papovaviruses have been observed to induce tumors only in experimental environments, typically when introduced into non-natural or foreign host species that they would not normally infect in nature.
In addition, oncogenic viruses vary significantly in their transforming efficiency. Some viruses are capable of initiating cellular transformation with just a single viral particle, whereas others require multiple particles to produce the same effect. They also differ in the proportion of cells that become transformed within a genetically susceptible population. As a result, laboratory studies reveal considerable variation in how effectively different viruses trigger tumor formation. Furthermore, certain transforming viruses exhibit specificity toward particular differentiated cell types, meaning their ability to induce cancer may depend on the nature and developmental state of the host cell they infect.
DNA Tumor Viruses
Certain DNA tumor viruses, including Adenovirus 12, Polyoma virus, and Simian virus 40, are known for their ability to rapidly transform susceptible cells in laboratory conditions. However, typically only a small fraction of exposed cells transform. Polyoma virus naturally circulates in both wild and laboratory mouse populations. In contrast, Simian virus 40 (SV40), while originally identified in monkeys, does not cause tumors in its natural primate host but can induce tumor formation in rodents under experimental conditions.
Another important DNA tumor virus, Epstein-Barr virus (EBV), a member of the herpesvirus group, has been strongly linked to several human cancers. Discovered in 1964 by Michael Anthony Epstein and Yvonne Barr in cultured cells from Burkitt lymphoma, EBV is also associated with Infectious mononucleosis, Hodgkin lymphoma, and Nasopharyngeal carcinoma. Additionally, Herpes simplex virus type 1 and Herpes simplex virus type 2 have been implicated in certain cancers, including malignancies of the oral region and cervical cancer, respectively.
Mechanism of Viral DNA Integration and Cellular Transformation
Cellular transformation by DNA tumor viruses often occurs when viral genetic material integrates into the host cell’s genome under conditions that prevent full viral replication. Normally, complete viral gene expression would lead to the production of new virus particles and eventual destruction of the host cell. However, in some cases, infection becomes non-productive, meaning that viral replication does not proceed to completion. Despite this, specific early viral genes necessary for initiating viral replication continue to be expressed. The persistent activity of these early viral gene products can disrupt normal cellular regulatory pathways. Over time, this unintended interference with cell growth control mechanisms may result in permanent transformation and the development of tumors.
Molecular Basis of Transformation by RNA Sarcoma Viruses
RNA sarcoma viruses are among the most potent transforming viruses identified. They can induce transformation in a wide range of cell types, including fibroblasts, myoblasts, and iris epithelial cells. In laboratory cultures, they are highly efficient and may transform nearly all susceptible cells present, demonstrating a much stronger transforming ability than many other oncogenic viruses.
The genetic basis of this powerful transformation has been clarified through studies of Rous sarcoma virus in infected chicken fibroblast cells. This virus carries a distinct transformation gene within its RNA genome that is not required for viral replication but is essential for initiating cellular transformation. Following infection, the viral RNA is converted into DNA, which includes the transformation gene along with other viral genes. This DNA integrates into a specific site within the host cell’s genome. Once integrated, the viral genes are expressed, producing proteins that drive both cell transformation and the production of new virus particles. The stable integration of the transformation gene into the host genome explains why Rous sarcoma virus has a much greater capacity to transform susceptible cells than most oncogenic DNA viruses.
Human Oncogenic Viruses and Cancer Prevention
Research over the past several decades has firmly established that certain viruses play a direct role in the development of human cancers. Among the most significant are Human papillomavirus (HPV), Hepatitis B virus (HBV), Hepatitis C virus (HCV), and Epstein-Barr virus (EBV). These viruses are linked to malignancies such as cervical carcinoma, hepatocellular carcinoma, certain lymphomas, and nasopharyngeal cancer (Table 1). They promote tumor formation through diverse mechanisms, including integration of viral DNA into host chromosomes, persistent expression of viral oncogenes, and long-term inflammation that disrupts normal cellular regulation.
Table 1
| Virus | Genome Type | Major Associated Cancers | Mechanism of Oncogenesis |
| Human papillomavirus (HPV) | dsDNA | Cervical cancer, Oropharyngeal cancer | Integration of viral DNA; E6 and E7 oncoproteins inactivate tumor suppressors |
| Hepatitis B virus (HBV) | dsDNA | Hepatocellular carcinoma | DNA integration; chronic inflammation; oncogenic viral proteins |
| Hepatitis C virus (HCV) | ssRNA | Hepatocellular carcinoma | Chronic inflammation; indirect genomic instability |
| Epstein-Barr virus (EBV) | dsDNA | Burkitt lymphoma, Hodgkin lymphoma, Nasopharyngeal carcinoma | Latent infection; expression of viral oncogenes |
| Human T-lymphotropic virus 1 (HTLV-1) | ssRNA (Retrovirus) | Adult T-cell leukemia/lymphoma | Viral Tax protein alters cell cycle regulation |
Conclusion
Viruses, once considered unlikely contributors to cancer, are now firmly established as significant yet often hidden drivers of malignancy. Both RNA and DNA viruses can interfere with normal cellular control mechanisms. They may integrate their genetic material into the host genome. Some produce oncogenic proteins that disrupt cell-cycle regulation. Others cause chronic inflammation that contributes to uncontrolled cell growth. Research on tumor viruses has greatly expanded our understanding of how normal cells become cancerous. It has been shown that some human cancers arise from preventable viral infections. The discovery of viral oncogenesis has broadened the concept of cancer beyond purely genetic causes. This insight has created new opportunities for vaccination, early diagnosis, and targeted treatment strategies.
You may also like:

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.