In this article, I briefly describe tumor immune escape mechanisms and apoptotic resistance. Tumor cells evade immune surveillance and resist apoptosis through multiple interconnected mechanisms, promoting survival and progression. Understanding these strategies is essential for advancing effective cancer immunotherapies.
The Dual Role of Immune Response
The immune system operates through a delicate balance of activating and inhibitory pathways. While insufficient activity can allow disease to progress, excessive or uncontrolled responses may trigger chronic inflammation and autoimmunity. Immune activity can provide effective defense against tumors, yet under certain conditions, inflammatory responses may instead support tumor initiation, growth, and survival.
Chronic Inflammation and Tumor Promotion
Persistent inflammation shapes a microenvironment that favors tumor development through multiple mechanisms. Prolonged immune activity generates cellular stress and genotoxic damage, elevating mutation rates and thereby driving tumorigenesis. In addition, cytokines and growth factors released by leukocytes stimulate proliferation; once genetic alterations arise, tumor cells may exploit these signals to enhance their own growth. Inflammatory processes are also strongly proangiogenic and prolymphangiogenic, fostering the expansion of blood and lymphatic vessels. This not only delivers oxygen and nutrients to the tumor mass but also facilitates invasion and metastatic spread through newly formed lymphatic channels. Collectively, these effects illustrate how chronic inflammation contributes to the hallmarks of cancer.
Tumor Promoting Antibodies and Blocking Factors
Antibodies directed against tumor-specific antigens were once expected to play a decisive role in tumor eradication. However, experimental evidence revealed that such antibodies can paradoxically promote tumor progression rather than suppress it. Attempts to inhibit tumor growth through active immunization with tumor antigens or the passive transfer of anti-tumor antibodies have frequently failed to protect the host, instead enhancing tumor development.
Early studies using cell-mediated lympholysis (CML) assays demonstrated that sera from animals with progressive tumors could inhibit the cytotoxic T lymphocyte (CTL)– mediated lysis of target cells, whereas sera from animals with regressing tumors lacked this inhibitory effect. Building on these observations, Ingegered and Karl Erik Hellström reported in 1969 that children with progressive neuroblastoma possessed elevated levels of serum-derived blocking factors, while such factors were absent in children whose tumors were regressing. Since then, similar serum blocking factors have been identified in association with various human cancers, underscoring their role in tumor immune evasion.
In certain instances, anti-tumor antibodies themselves function as serum blocking factors by binding to tumor-specific antigens and concealing them from recognition by cytotoxic T cells. More commonly, however, the blocking factors are not antibodies alone but immune complexes formed between antibodies and free tumor antigens. While such complexes are known to suppress CTL activity, the precise mechanism underlying this inhibition remains unclear. Additionally, these immune complexes can impair antibody-dependent cellular cytotoxicity (ADCC) by occupying Fc receptors on natural killer (NK) cells and macrophages, thereby preventing their effective engagement with tumor targets.
Immunosuppressive Tumor Microenvironment
Tumor and immune cells can secrete soluble mediators that shape the local tumor milieu into an immunosuppressive environment. Researchers have documented elevated levels of molecules such as transforming growth factor-β (TGF-β) and indoleamine-2,3-dioxygenase (IDO), both of which suppress TH1 responses, in several human cancers. Interleukin-10 (IL-10) adds further complexity. In some contexts, it fosters tolerance and tumor progression, while in others, it promotes innate immune responses with antitumor potential. Similarly, TGF-β appears to play a stage-dependent role. It acts as a tumor suppressor during early growth but drives tumor progression in later stages by inhibiting dendritic cell activation and impairing T-cell function. Notably, these effects are often confined to the immediate tumor site.
Experimental studies in mice illustrate this localization. Although primary tumors persisted, the immune system rejected additional cancer cells of the same type introduced at a separate site, highlighting the critical influence of the tumor microenvironment. Both animal models and human studies further highlight the role of immunosuppressive cell populations. It includes regulatory T cells (TREGs), myeloid-derived suppressor cells (MDSCs), M2-polarized macrophages, and TH2 cells. Collectively, their presence skews the immune balance away from effective TH1-mediated responses, enabling tumors to evade immune surveillance and continue progressing.
Tumor Escape and Apoptosis
Although the immune system is capable of recognizing and responding to tumor cells that display tumor-associated antigens, the high incidence of cancer-related deaths underscores the frequent ineffectiveness of these responses. The continuous selective pressure exerted by antitumor immunity favors the survival of escape variants—tumor cells that acquire the ability to avoid immune recognition and destruction. Immune surveillance actively shapes tumor evolution, while tumors adapt to evade it, a process known as immunoediting.
Impaired Antigen Presentation and Tumor Progression
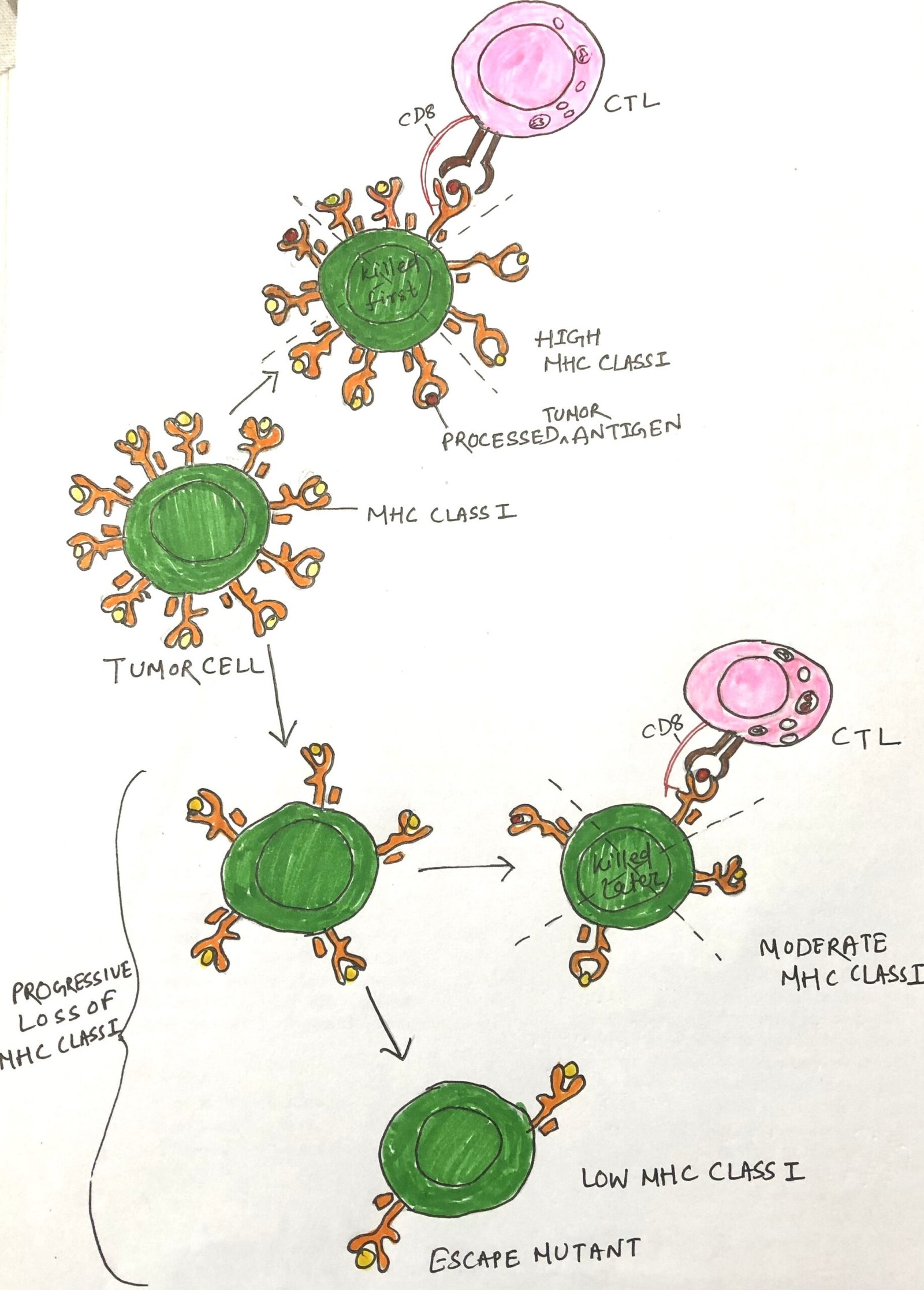
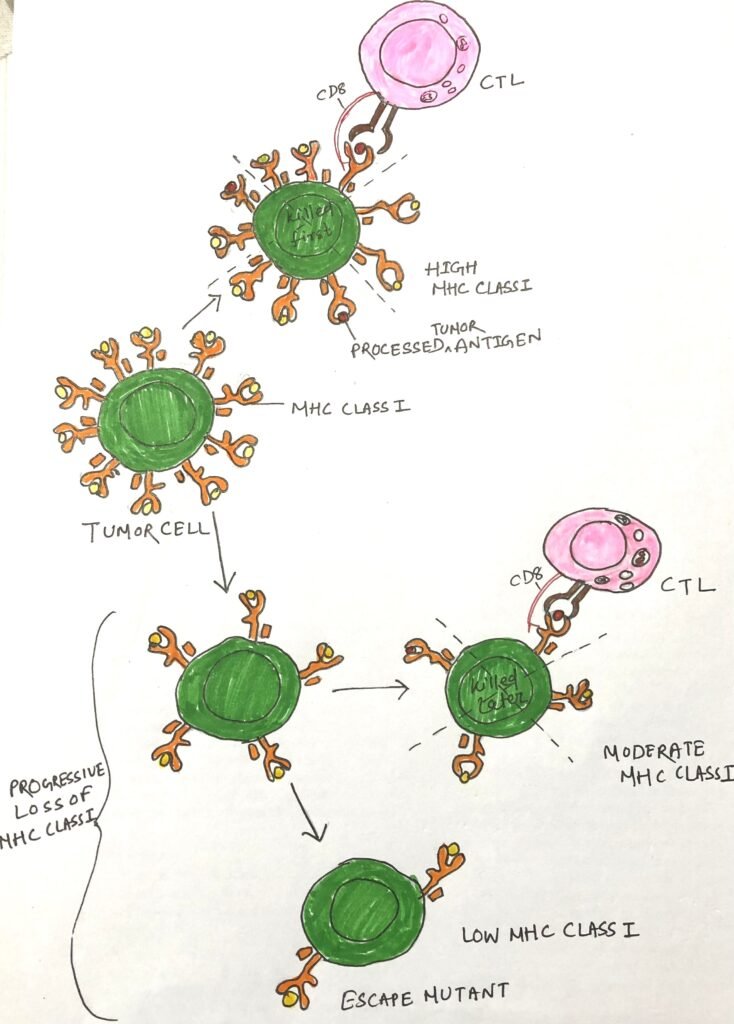
Many tumor escape variants exhibit abnormalities in antigen processing and presentation, which hinder effective immune recognition. These defects may include reduced expression of MHC molecules, secretion of tumor-specific antigens (TSAs), mutations affecting the transporter associated with antigen processing (TAP) or β2-microglobulin, and insensitivity to interferon-γ (IFN-γ). Each of these alterations diminishes MHC class I presentation of tumor antigens, significantly impairing CD8+ T cell–mediated recognition (Figure 1). Many tumors simultaneously downregulate ligands for activating NK cell receptors, preventing natural killer (NK) cells from targeting cells that lack MHC class I. Thus, evading NK cell cytotoxicity. Tumor cells often lose or reduce MHC molecules, which generally correlates with disease progression and indicates a poor clinical prognosis.
Evasion of Apoptosis in Cancer Progression
Neoplastic cells often acquire resistance to programmed cell death, or apoptosis, disrupting the balance between cell proliferation and death and allowing expansion of the tumor population. This resistance can arise from alterations in extrinsic pathways, such as defects in cell-surface death receptors, as well as intrinsic mitochondrial apoptosis mechanisms. For example, Fas (CD95) and TRAIL receptors, along with NKG2D-mediated signaling from NK cells, transmit external apoptotic cues. Any of these pathways having impairments can block apoptosis. Similarly, changes in mitochondrial regulators of cell death, such as decreased pro-apoptotic factors or increased anti-apoptotic proteins, enhance tumor cell survival and promote cancer progression. Furthermore, defective DNA repair mechanisms in transformed cells, combined with selective pressure from the immune system, facilitate the accumulation of tumor cells harboring these survival-promoting mutations.
Poor Co-stimulatory Signals and Immunosuppressive Microenvironments
Full activation of T cells requires two signals. The first one arises from recognition of a peptide–MHC complex by the T-cell receptor. The second is a co-stimulatory signal, typically provided through interactions between CD80/86 (B7) on antigen-presenting cells and receptors such as CD28 on T cells. Both signals are essential for IL-2 production and T-cell proliferation. Tumor cells are self-derived somatic cells.
They generally exhibit low immunogenicity and often lack sufficient costimulatory molecules. In the absence of an adequate number of antigen-presenting cells and necessary stimulatory signals in the tumor microenvironment, T cells may receive only partial activation, which can lead to clonal anergy and immune tolerance. Moreover, unactivated T cells interacting with tumor cells can differentiate into immunosuppressive phenotypes, such as regulatory T (TREG) cells, producing inhibitory cytokines and employing co-inhibitory molecules like CTLA-4 to impede antitumor immunity. Notably, several modern cancer therapies aim to overcome these limitations by enhancing T-cell co-stimulation to restore effective antitumor responses.
Conclusion
A combination of immune evasion strategies and survival adaptations drives tumor progression. Chronic inflammation and tumor-promoting antibodies establish a microenvironment that supports tumor growth while inhibiting effective immune responses. Immunosuppressive cells and soluble factors within the tumor microenvironment further dampen antitumor immunity, creating a localized state of immune tolerance. Tumor cells also acquire defects in antigen processing and presentation, such as reduced MHC class I expression, which limit recognition by cytotoxic T cells and allow evasion of natural killer cell surveillance. Finally, resistance to apoptosis and impaired T-cell activation, including the induction of regulatory T cells, enable tumor cells to survive, proliferate, and escape immune-mediated elimination. Collectively, these interconnected mechanisms highlight the complex interplay between tumors and the immune system and underscore the importance of therapeutic strategies that restore effective antitumor immunity.
You may also like:
- Costimulatory receptors for activation of T cells
- The mechanism of killing of target cells by effector cells
- Immunotherapies Redefining Cancer Treatment

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.