In this article, I briefly describe triacylglycerols, the fatty acid esters of glycerol. Triacylglycerols store energy and provide insulation. Partial hydrogenation of edible oils creates fatty acids with harmful side effects.
Structure and Properties of Triacylglycerol
Triacylglycerols, or triglycerides, fats, or neutral fats, are the simplest lipids derived from fatty acids. They consist of three fatty acid molecules, each linked to a single glycerol molecule (Figure 1) through ester bonds. When all three fatty acids are identical, the resulting molecule is called a simple triacylglycerol, named after the specific fatty acid present. Examples include tripalmitin (16:0), tristearin (18:0), and triolein (18:1). However, most natural triacylglycerols are mixed, containing different types of fatty acids. Due to the ester linkages that bind the glycerol’s hydroxyl groups to the fatty acids’ carboxyl groups, triacylglycerols are nonpolar, hydrophobic, and virtually insoluble in water. Additionally, because lipids have a lower specific gravity than water, oil and water mixtures separate into two layers, with oil floating above the water phase.
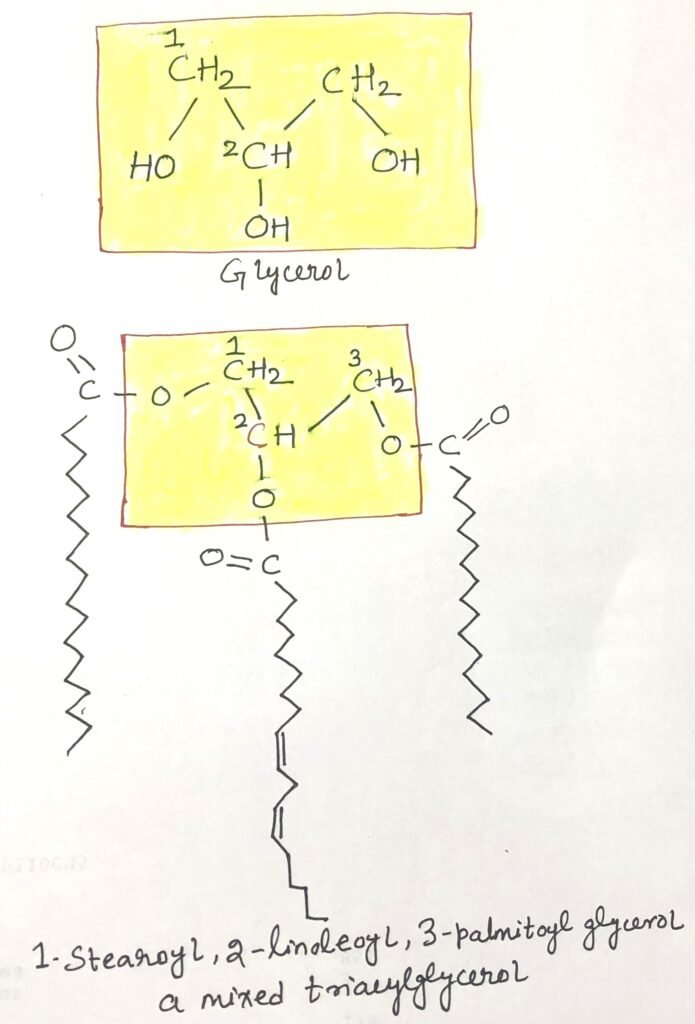
Triacylglycerols Aid in Efficient Energy Storage and Insulation
In the aqueous cytosol, triacylglycerols separate into microscopic oil droplets, functioning as reservoirs of metabolic fuel. Specialized fat cells, known as adipocytes, house a myriad of triacylglycerols in droplets that almost entirely crowd the cell’s interior in vertebrates. Many plants store triacylglycerols as oils in the seeds. This aids in providing energy and biosynthetic precursors during seed germination. Adipocytes and germinating seeds produce lipases—enzymes that break down stored triacylglycerols to release fatty acids. These fatty acids are then transported to areas to generate the required energy.
Triacylglycerols offer two key advantages over polysaccharides as energy storage molecules. First, fatty acids have more reduced carbon atoms than sugars. Thus, their oxidation generates more than double the energy per gram relative to carbohydrates. Second, triacylglycerols are hydrophobic and do not associate with water, meaning that organisms storing fat avoid the additional weight that comes with the water bound to stored polysaccharides.
In humans, fat tissue, mainly made up of adipocytes, is found beneath the skin, within the abdominal cavity, and in the mammary glands. People who are moderately overweight, carrying around 15 to 20 kg of triacylglycerols in their adipose tissue, could sustain their energy requirements for several months using these fat reserves. On the other hand, the body can store only enough glycogen to support less than a day’s energy needs. Despite this, carbohydrates like glucose have advantages as rapid energy sources, largely due to their high solubility in water.
In some animals, triacylglycerols act as energy reserves and provide insulation against cold climates. Polar animals such as seals, walruses, and penguins have thick layers of fat for warmth. Similarly, hibernating animals build up large fat stores before going dormant, which serve to insulate and to supply energy throughout the hibernation period.
Health Risks of Partially Hydrogenated Cooking Oils
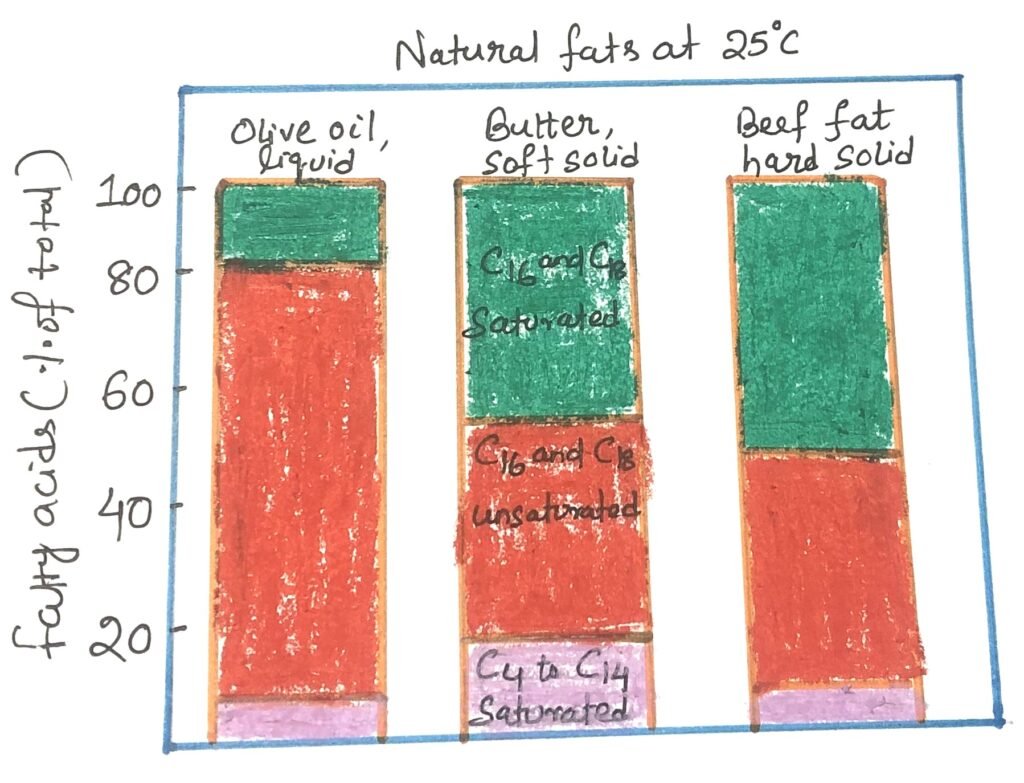
Vegetable oils, dairy products, and animal fats are complex mixtures containing both simple and mixed triacylglycerols. These contain various fatty acids that differ in chain length and saturation level (Figure 2). Vegetable oils like corn oil and olive oil are rich in unsaturated fatty acid-containing triacylglycerols. So, they remain liquid at room temperature. In contrast, triacylglycerols made up solely of saturated fatty acids, such as tristearin, a primary component of beef fat, are solid and greasy at room temperature.
Prolonged exposure of lipid-rich foods to air can lead to spoilage. The unpleasant taste and smell of rancid fats result from the oxidation of double bonds in unsaturated fatty acids, generating shorter-chain aldehydes and carboxylic acids. These volatile compounds readily disperse in the air, making the rancid odor easily detectable. To extend the shelf life of edible vegetable oils and improve their heat stability for deep frying, these oils undergo partial hydrogenation. This process transforms many of the cis double bonds in fatty acids into single bonds. Thus, it raises the melting point of the oils and makes them solid at room temperature. However, a negative side effect of this method is the unintended conversion of some cis double bonds into trans double bonds.
Consuming trans fats in the diet is linked to an increased risk of cardiovascular disease. It should be minimized to help prevent coronary heart disease. Trans fatty acids contribute to higher levels of triacylglycerols and low-density lipoprotein (LDL or “bad cholesterol”) in the bloodstream, while reducing levels of high-density lipoprotein (HDL or “good cholesterol”). They also promote inflammation in the body.
Conclusion
Triacylglycerols are lipids composed of three fatty acids attached to a glycerol molecule through ester bonds. They may be simple, with identical fatty acids, or mixed, containing different types. These molecules are nonpolar and hydrophobic, making them insoluble in water. Because of their lower density, they float when mixed with water.
In the cytosol, triacylglycerols form oil droplets that act as energy reserves. In animals, they are stored in adipocytes, where the droplets fill most of the cell’s interior. Plants store them in seeds to provide energy during germination. Lipase enzymes break down these triacylglycerols, releasing fatty acids that are transported to where they’re needed for energy.
People who are moderately overweight, carrying around 15 to 20 kg of triacylglycerols in their adipose tissue, could potentially sustain their energy requirements for several months using these fat reserves. In some animals, triacylglycerols not only act as energy reserves but also provide insulation against cold climates. Polar animals such as seals, walruses, and penguins have thick layers of fat for warmth.
Lipid-rich foods spoil when exposed to air due to oxidation of unsaturated fats, producing foul-smelling compounds. To extend shelf life and improve heat stability, oils are partially hydrogenated, turning cis double bonds into single bonds. However, this process can also create harmful trans fats. Consuming trans fats in the diet is linked to an increased risk of cardiovascular disease and should be minimized to help prevent coronary heart disease.
You may also like:
- The metabolism of fuel in fasting and starvation
- Eicosanoids and steroid hormones carry messages to cells and tissues
- Cholesterol Transport and Lipoprotein Metabolism

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.




