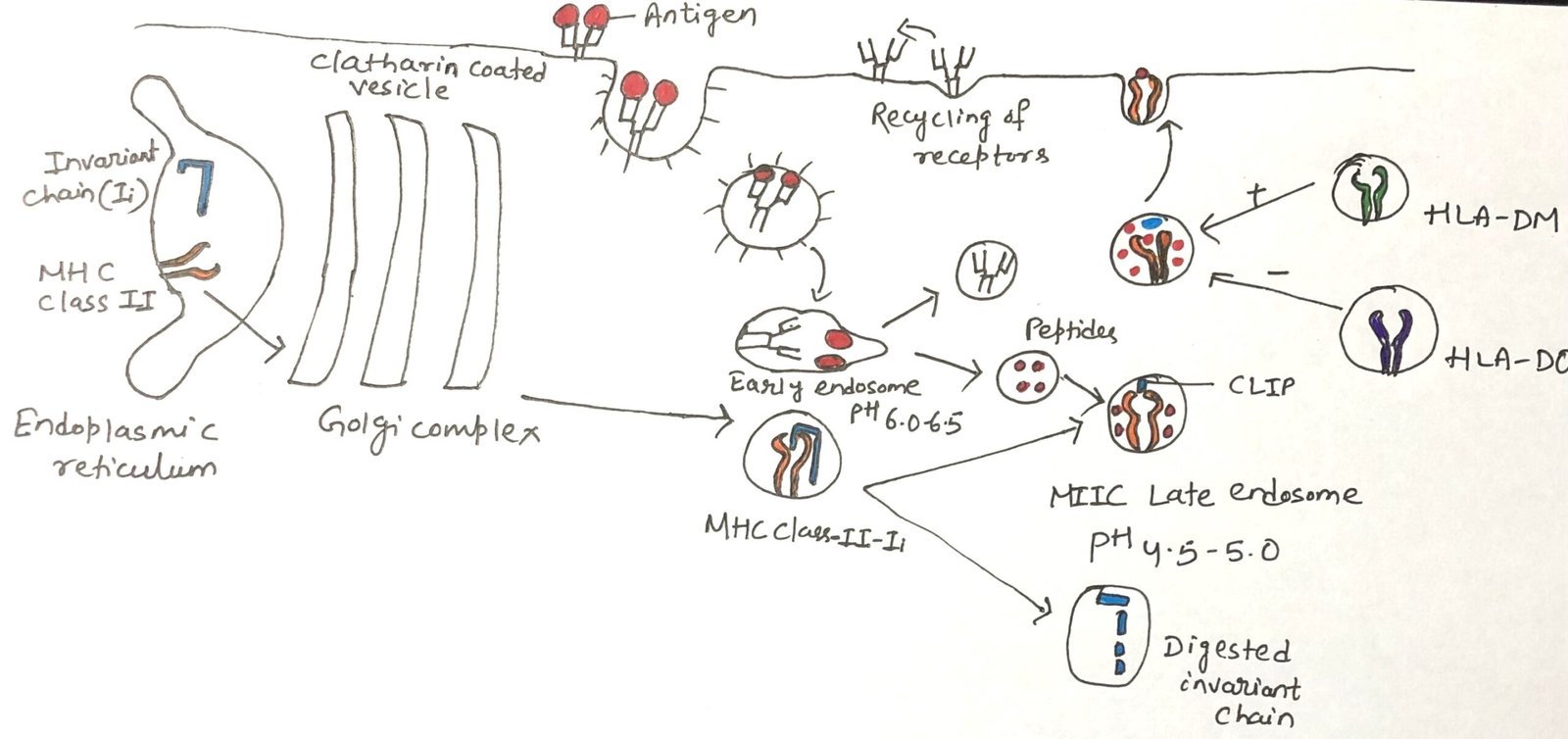
In this article, I briefly describe the role of the invariant chain in guiding the transport of class-II MHC molecules from the rough endoplasmic reticulum to late endosomes. It plays a role in immune defense.
The Invariant Chain: An essential chaperone in antigen presentation
The immune system functions through a finely tuned interplay of molecules that identify and remove pathogens. Within this network, the invariant chain (Ii) plays a pivotal role, directing MHC class II molecules to endocytic vesicles where they encounter antigenic peptides. In the absence of this chaperone, the presentation of antigens to CD4⁺ T cells becomes inefficient, ultimately compromising the adaptive immune response.
Antigen-presenting cells display both MHC class I and class II molecules. Newly synthesized MHC molecules of each class are initially located together in the membrane of the rough endoplasmic reticulum (RER). To avoid inappropriate binding of peptides meant for MHC class I, the MHC class II molecules associate with a specialized protein known as the invariant chain (Ii or CD74) during their synthesis in the RER.
The invariant chain is a conserved, non-MHC encoded protein that associates with the peptide-binding groove formed by the α and β chains of MHC class II. By occupying this groove, it prevents premature binding of endogenous peptides while the class II complex is still within the rough endoplasmic reticulum (RER) (Figure 1). Additionally, the invariant chain assists in the proper folding of the α and β chains, facilitates their exit from the RER, and directs MHC class II molecules through the trans-Golgi network to the endocytic pathway for antigen processing.
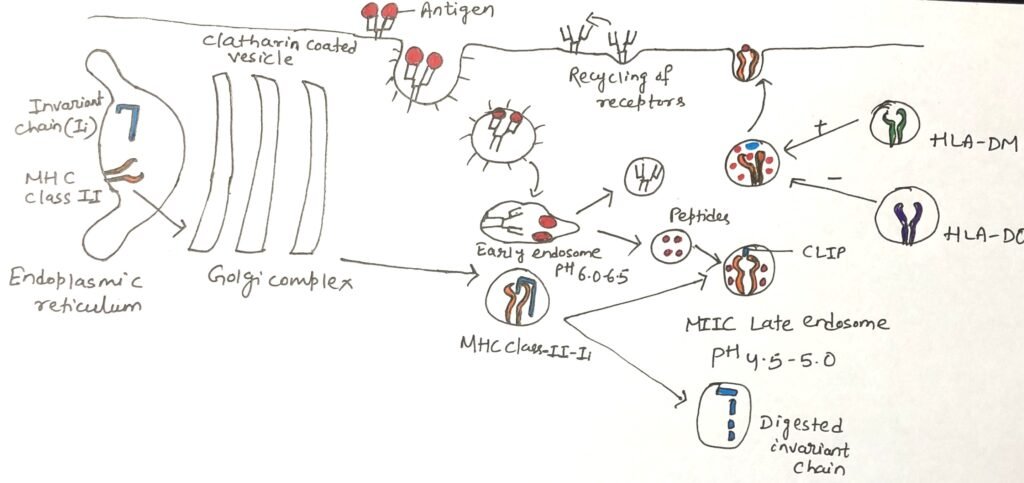
The Invariant Chain Serves as a Chaperone
In cells lacking both MHC class II and invariant chain genes, transfection with only MHC class II genes results in the molecules being retained within the endoplasmic reticulum. Immunofluorescence studies show that, without the invariant chain, these class II molecules fail to progress beyond the cis-Golgi and cannot reach their proper destination in vesicles.
In contrast, when cells are transfected with both MHC class II and invariant chain genes, the class II molecules are correctly directed to vesicular structures within the endocytic pathway. This trafficking is guided by sorting signals located in the cytoplasmic tail of the invariant chain, which ensures transport of the MHC class II complex from the trans-Golgi network to endocytic compartments.
Processing of MHC Class II–Invariant Chain Complexes
MHC class II molecules associate with the invariant chain in the rough endoplasmic reticulum, and these complexes are transported through the Golgi apparatus and trans-Golgi network. They then follow the endocytic pathway, progressing from early endosomes to the MIIC late endosomal compartment.
As they move through these compartments, increasing proteolytic activity leads to stepwise degradation of the invariant chain. A small portion of it, called CLIP (Class II-associated invariant chain peptide), remains attached within the peptide-binding groove of the MHC class II molecule. CLIP functions to temporarily block the groove, preventing the premature loading of antigenic peptides until the appropriate stage of processing.
Role of HLA-DM in Antigen Presentation
A specialized, non-classical MHC class II molecule known as HLA-DM plays a key role in replacing CLIP with antigenic peptides (Figure 1). The genes encoding its two chains, DMα and DMβ, are located within the human MHC region, near the TAP and LMP genes; mice possess similar gene counterparts.
Like classical class II molecules, HLA-DM is a heterodimer of α and β chains. However, it differs in two important ways: it shows very little polymorphism and is not typically expressed on the cell surface. Instead, HLA-DM resides mainly in endosomal compartments. Within these compartments, HLA-DM interacts with the β chain of classical MHC class II molecules. Its function is to remove unstable peptides, including CLIP, from the peptide-binding groove, a process often described as peptide editing. Only peptides that bind strongly and form stable complexes with MHC class II molecules are resistant to displacement by HLA-DM. These stable MHC–peptide complexes are the ones that eventually reach the cell surface for presentation to T cells.
Stability of MHC Class II–Peptide Complexes
Similar to MHC class I molecules, MHC class II molecules rely on peptide binding for their proper structure and stability. Once a peptide is loaded, the MHC class II–peptide complex is transported to the cell surface. At the neutral pH of the plasma membrane, the complex adopts a compact and stable conformation. In this form, the peptide is bound so tightly that replacing it with another peptide under normal physiological conditions is highly unlikely.
Another non-classical MHC class II molecule, HLA-DO, is also relatively non-polymorphic and forms associations with classical MHC class II molecules. Unlike HLA-DM, however, HLA-DO functions as a negative regulator of antigen presentation. It modulates HLA-DM activity, thereby shaping the set of peptides that are preferentially loaded onto classical class II molecules. In cells expressing both HLA-DM and HLA-DO, the two molecules form a stable complex in the endoplasmic reticulum and remain associated as they traffic to endosomal compartments, where they jointly influence peptide selection.
HLA-DO and Its Role in Self-Tolerance
HLA-DO expression was first identified in B cells and the thymus but has more recently been observed in dendritic cells, where it appears to contribute to the maintenance of self-tolerance. This role was highlighted in a study by L. K. Denzin and colleagues, who used diabetes-prone mice engineered to express human HLA-DO. Dendritic cells are central to establishing self-tolerance and presenting autoantigens to self-reactive T cells.
In non-obese diabetic mice, self-reactive T cells normally arise and destroy pancreatic β-cells, leading to type 1 diabetes. However, in this study, the presence of the HLA-DO transgene in dendritic cells prevented the onset of diabetes. Further analysis with specific monoclonal antibodies revealed that HLA-DO expression altered the peptide repertoire presented to autoreactive T cells. As a result, the efficiency of presenting key self-peptides was reduced. These findings suggest that normal HLA-DO activity fine-tunes the function of HLA-DM, promoting the display of self-peptides in a way that supports immune tolerance and helps prevent autoimmunity.
Conclusion
The immune system functions through a finely tuned interplay of molecules that identify and remove pathogens. Within this network, the invariant chain (Ii) is pivotal, directing MHC class II molecules to endocytic vesicles where they meet antigenic peptides.
Antigen-presenting cells display both MHC class I and class II molecules. To avoid inappropriate binding of peptides meant for MHC class I, the MHC class II molecules associate with a specialized protein known as the invariant chain (Ii or CD74) during their synthesis in the RER.
The invariant chain is a conserved, non-MHC encoded protein that associates with the peptide-binding groove formed by the α and β chains of MHC class II. It assists in the proper folding of the α and β chains, facilitates their exit from the RER, and directs MHC class II molecules through the trans-Golgi network to the endocytic pathway for antigen processing. Immunofluorescence studies show that, without the invariant chain, these class II molecules fail to progress beyond the cis-Golgi and cannot reach their proper destination in vesicles.
MHC class II molecules associate with the invariant chain in the rough endoplasmic reticulum, and these complexes are transported through the Golgi apparatus and trans-Golgi network. A specialized, non-classical MHC class II molecule known as HLA-DM plays a key role in replacing CLIP with antigenic peptides. HLA-DO expression was first identified in B cells and the thymus but has more recently been observed in dendritic cells, where it appears to contribute to the maintenance of self-tolerance.
You may also like:

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.