In this article, I briefly describe nucleic acid vaccines, their mechanism of working, and the broad and long-term immune response in humans.
Nucleic acid vaccines
The vaccines which use genetic material from a harmful pathogen to stimulate an immune response are called nucleic acid vaccines. These vaccines can contain either DNA or RNA. This genetic material serves as a blueprint for producing a specific protein from the pathogen, recognized as an antigen by the immune system. Once delivered into host cells, the genetic material is processed by the cell’s protein-making machinery to produce antigens, activating the immune response. This approach offers several potential advantages, such as the ability to induce robust and long-lasting immunity, excellent stability, and relatively straightforward large-scale production.
DNA vaccines
DNA plasmid vaccines consist of a small, circular DNA molecule called a plasmid, which carries genes encoding proteins from a specific pathogen. When the plasmid DNA is injected directly into muscle tissue, the cells absorb it and produce the encoded protein antigen. This triggers both a humoral (antibody-mediated) and a cell-mediated immune response (figure 1). The DNA may either integrate into the host’s chromosomal DNA or persist in the cell as an episome for extended periods. The viral antigen is expressed by muscle cells and nearby dendritic cells that take up the plasmid DNA and produce the antigen.
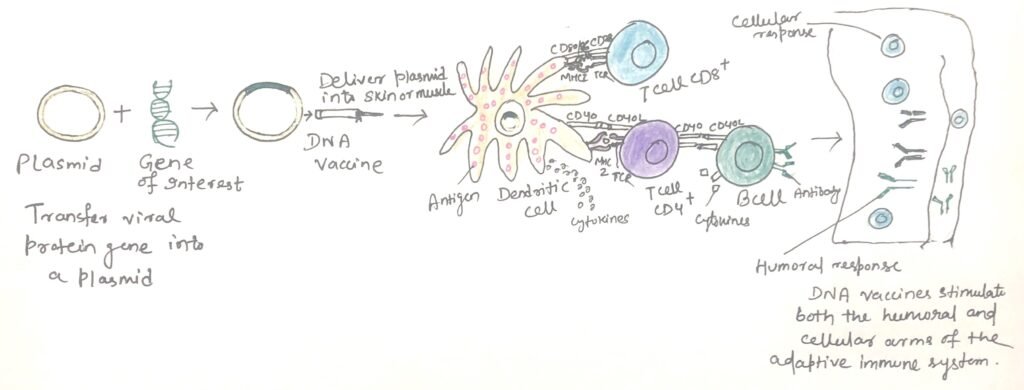
A key advantage of DNA vaccines is that the host expresses the antigen naturally. Thus, it elicits an immune response that closely mirrors how the actual pathogen presents the antigen. Unlike traditional vaccines, which often rely on live attenuated pathogens and come with associated risks, DNA vaccines stimulate both arms of the immune system, inducing both humoral and cell-mediated immunity.
The protein encoded by the DNA is processed through multiple pathways. Peptides derived from the protein are presented as endogenous antigens on the surface of muscle and antigen-presenting cells via MHC class I molecules, activating cytotoxic T cells. Additionally, the protein is also expressed in a soluble, secreted form, which is processed and presented via MHC class II molecules. This leads to the activation of B cells (figure 1), the production of antibodies, and the generation of B-cell memory. DNA vaccines also promote long-term immunological memory due to the sustained expression of the antigen.
Advantages and disadvantages
Plasmid DNA vaccines offer the advantage of not requiring refrigeration. This significantly reduces the cost and logistical challenges of vaccine distribution. Also, the same manufacturing methods can be applied to produce various DNA vaccines. Each of them is designed to encode antigens from different pathogens. To facilitate delivery, the plasmid DNA is coated onto microscopic gold particles. These coated particles are administered through the skin into the underlying muscle using a gene gun. This enables rapid vaccine delivery without relying on large quantities of needles and syringes.
DNA vaccines have demonstrated the ability to induce protective immunity against several pathogens, including the influenza virus. As of March 2018, no DNA vaccines have been licensed for human use. However, one DNA vaccine has been approved for veterinary use—a West Nile virus vaccine for horses. Currently, the most promising and farthest along of the human trials of a DNA vaccine are actually for cancer treatment.
However, DNA vaccines have some limitations. They are restricted to protein-based antigens and are ineffective for non-protein antigens like bacterial polysaccharides. There is also a risk of typical processing when dealing with bacterial or parasite proteins. Furthermore, when plasmid DNA nanoparticles are delivered via nasal sprays, there is a potential risk of transfecting unintended cells, such as brain cells.
RNA vaccines
An RNA vaccine, also known as an mRNA (messenger RNA) vaccine, leverages a copy of messenger RNA (mRNA), a naturally occurring molecule, to elicit an immune response. Unlike traditional vaccines, which introduce a weakened or inactivated form of a germ into the body, mRNA vaccines teach cells to produce a specific protein that triggers an immune reaction. These vaccines deliver synthetic RNA molecules into immune cells, where the RNA acts as mRNA. This prompts the cells to create a foreign protein that would typically be associated with a pathogen (like a virus) or a cancer cell. These proteins then activate an adaptive immune response, training the body to recognize and destroy the corresponding pathogen or cancer cells.
RNA vaccines offer several advantages over traditional protein-based vaccines. This includes faster and more efficient design and production, lower manufacturing costs, and the ability to induce cellular and humoral immunity. The mRNA is delivered through a co-formulation with lipid nanoparticles, which safeguard the RNA strands and enhance their uptake by cells.
The development and impact of mRNA vaccines
Although mRNA vaccines are relatively new, researchers have been studying and refining them for decades. These vaccines can be developed in laboratories using readily available materials, allowing the process to be standardized and scaled up efficiently. This enables much faster vaccine development compared to traditional methods. Before the COVID-19 pandemic, mRNA vaccines had already been studied for diseases such as influenza, Zika virus, rabies, and cytomegalovirus (CMV).
When the genetic information of the virus causing COVID-19 became available, scientists quickly designed mRNA instructions to prompt cells to produce the virus’s unique spike protein. This innovation drew significant attention in the field of RNA therapeutics. By December 2020, two groundbreaking mRNA-based COVID-19 vaccines had completed the required eight-week post-trial evaluation and were awaiting emergency use authorization (EUA): Moderna’s mRNA-1273 and Pfizer–BioNTech’s BNT162b2.
The Pfizer-BioNTech vaccine received EUA from the U.S. Food and Drug Administration (FDA) on 11 December 2020, and the Centers for Disease Control and Prevention (CDC) recommended its use for individuals aged 16 and older on 12 December. Shortly after, on 19 December 2020, the CDC endorsed the use of Moderna’s vaccine in adults following its FDA EUA approval.
The working of mRNA vaccines
These vaccines deliver a temporary, synthetically produced segment of the virus’s RNA to the recipient. This RNA is absorbed by dendritic cells (specialized immune cells) through phagocytosis. Inside these cells, the mRNA is processed by their ribosomes, which translate it into viral antigens encoded by the mRNA. The mRNA is then broken down. Dendritic cells are particularly efficient at taking in the mRNA particles compared to other non-immune cells.
After the host cell produces the viral antigens, the adaptive immune system takes over. Proteasomes break down the antigens, which are then bound to class I and class II MHC molecules. These are transported to the cell membrane, activating the dendritic cell. Once activated, dendritic cells travel to the lymph nodes, where they present the antigen to T cells and B cells. This interaction triggers the production of antibodies that are specifically designed to target the antigen, ultimately leading to immunity.
Role of lipid nanoparticles
Researchers have noticed that the mRNA COVID-19 vaccines are the most effective and efficient in younger individuals than older adults. The researchers from Drexel University’s College of Medicine and the Perelman School of Medicine at the University of Pennsylvania investigated the role of lipid nanoparticles (LNPs), a crucial element of these vaccines that facilitates the delivery of mRNA to cells, including those involved in the innate immune system (or immune response cells).
These vaccines rely on a vital lipid-based packaging system (figure 2) to protect and transport the mRNA into cells. This packaging consists of lipid nanoparticles (LNPs), which are typically made up of four types of lipids. One of these lipids becomes positively charged in mildly acidic conditions, enabling it to encapsulate the negatively charged mRNA during the vaccine preparation process. Other components of LNPs include cholesterol—the same type found in our bodies and in food—as well as molecules that provide structure and maintain particle stability. Once the LNPs enter cells, they release the mRNA.
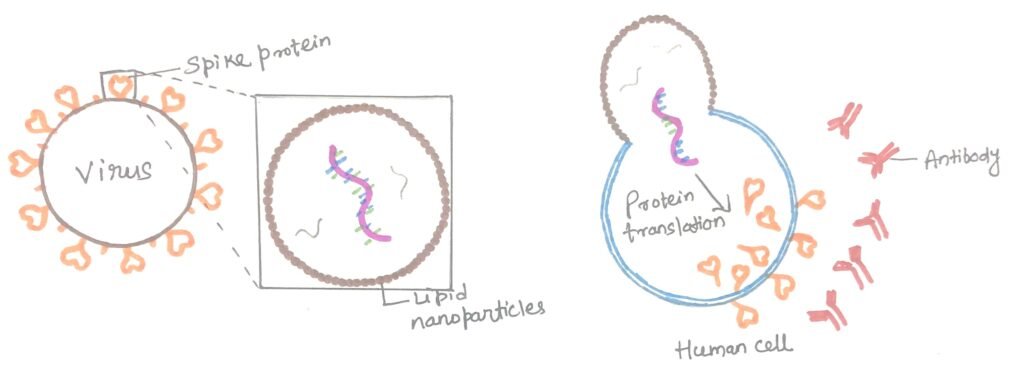
According to the researchers, LNPs play a key role in mRNA-based vaccines by aiding in the initiation of the immune response. A weaker response to LNPs in older adults may account for their reduced vaccine effectiveness, emphasizing the importance of enhancing these responses in the aging population.
Advantages and disadvantages
The production of mRNA vaccine is easier than antigen proteins or attenuated viruses. These vaccines provide both cellular and humoral immunity, and their design and production process is much faster than traditional vaccines. For instance, Moderna developed their mRNA-1273 COVID-19 vaccine in just two days.
Importantly, mRNA vaccines do not alter or interfere with DNA inside the cell. The synthetic mRNA used in the vaccine is a copy of a specific segment of the viral RNA that encodes instructions to produce the virus’s antigen (such as the spike protein in the case of COVID-19), and it has no connection to human DNA.
However, mRNA vaccines also have some drawbacks. Their reactogenicity is comparable to that of traditional, non-RNA vaccines. Individuals prone to autoimmune responses may experience adverse reactions. The mRNA strands in the vaccine can sometimes trigger an unintended immune response. Trials of COVID-19 mRNA vaccines reported strong but temporary reactogenic effects. Additionally, because mRNA is highly fragile, the vaccine must be stored at extremely low temperatures to prevent degradation, which could otherwise reduce its effectiveness in providing immunity.
Conclusion
Nucleic acid vaccines use genetic material from a harmful pathogen to stimulate an immune response. These vaccines can contain either DNA or RNA. This genetic material serves as a blueprint for producing a specific protein from the pathogen, recognized as an antigen by the immune system.
DNA plasmid vaccines consist of a small, circular DNA molecule called a plasmid, which carries genes encoding proteins from a specific pathogen. A key advantage of DNA vaccines is that the antigen is expressed in the host in its natural form, eliciting an immune response that closely mirrors how the antigen is presented by the actual pathogen. However, DNA vaccines are restricted to protein-based antigens and are not effective for non-protein antigens like bacterial polysaccharides.
mRNA vaccines teach cells to produce a specific protein that triggers an immune reaction. These proteins then activate an adaptive immune response, training the body to recognize and destroy the corresponding pathogen or cancer cells.
The production of mRNA vaccine is easier than antigen proteins or attenuated viruses. These vaccines provide both cellular and humoral immunity, and their design and production process is much faster than traditional vaccines. However, individuals prone to autoimmune responses may experience adverse reactions. The mRNA strands in the vaccine can sometimes trigger an unintended immune response. As mRNA is highly fragile, the vaccine must be stored at extremely low temperatures to prevent degradation.
You may also like:
- Recombinant vector vaccines retain many advantages of live attenuated vaccines
- Subunit vaccines– vaccines from purified macromolecules
- Improvement in vaccine immunogenicity and enhancing immune response

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.



