In this article, I briefly describe the negative regulation of B-cell activation.
B-cell activation and control
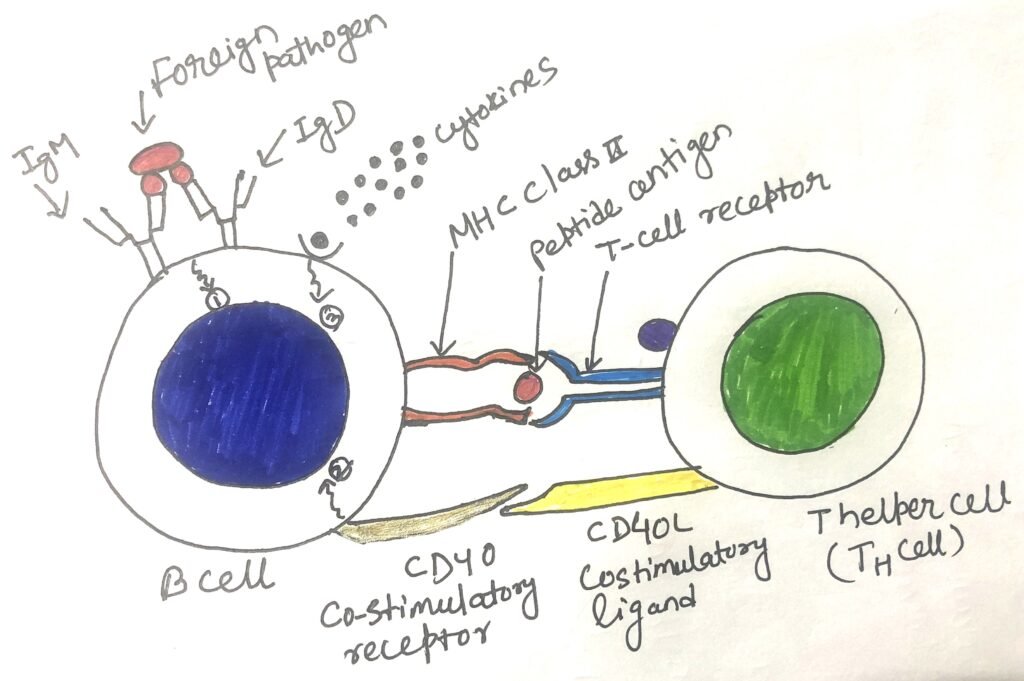
Presentation of antigens via MHC and receiving co-stimulatory signals (CD40-CD40L) from TH2 cells, results in the activation of B cells (figure 1). Following activation, B cells undergo cycles of mutation and selection to produce high-affinity memory B cells and plasma cells. Antigenic stimulation of B cells results in rapid proliferation, which needs to be controlled. Thus, once sufficient B cells are generated, it is necessary to halt the B-cell proliferation. Once the pathogen has been eliminated, most B-cells enter into an apoptotic program. This is also known as negative regulation of B-cell activation.
Transmembrane molecule CD22 gives negative signaling
The BCR (B-cell receptor) of activated mature B cells is associated with CD19/CD21 and CD81. In addition to this, the BCR of mature B cells is also associated with an extra transmembrane molecule, CD22. It is a cell-surface receptor molecule that recognizes N-glycolylneuraminic acid residues on serum glycoproteins and other cell surfaces. Thus, it can be doubled as an adhesion molecule.
CD22’s cytoplasmic domain bears an immunoreceptor tyrosine-based inhibitory motif (ITIM). ITIMs are the same as ITAMs, but they are inhibitory in function. B cell activation results in the phosphorylation of tyrosine in the CD22 ITIM. This allows the association of the SHP-1 tyrosine phosphatase with the cytoplasmic tail of CD22. SHP-1 then removes activating phosphatases from the tyrosine residues of neighboring signaling molecules.
Antigen engagement keeps the BCR signaling pathway active leading to the reattachment of phosphate groups to the tyrosine residues of adapter molecules and other signaling intermediates as fast as removing phosphate groups. Receptor-associated tyrosine kinase activity begins to slow down with the decrease in antigen levels. Then, signaling through CD22 can induce the removal of any residual activating phosphates. CD22 acts as a negative regulator of B-cell activation and its presence ensures the shutdown of signal from BCR when there is no antigen bound to it. CD22-/- mice have fewer marginal zone B cells than wild-type mice and are less responsive to TI antigens. Recent research also indicates that CD22-/- can’t generate GC (germinal center)-dependent memory B cells.
Receptor CD32 inhibits B-cell activation
CD32, also known as receptor FcγRIIb, and like CD22, it bears a cytoplasmic ITIM domain. It recognizes immune complexes containing IgG antibodies. B-cell activation is hindered by the binding of circulating antigen-IgG complexes to the B-cell surface. When B cell’s FcγRIIb molecules are attached to the BCR by a specific antigen-antibody immune complex, the FcγRIIb signaling cascade gets activated. The activation of the FcγRIIb cascade leads to the phosphorylation of FcγRIIb’s ITIM, which results in the inhibition of B-cell signaling.
CD5 negatively regulates B-cell signaling
CD5 acts as a negative regulator in both BCR and TCR signaling pathways. Experiments involving the removal of CD5 expression on B-1a B cells induce hyper-responsiveness to BCR-bound antigens. It suggests that CD5 acts as a negative regulator of BCR signaling.
B cell subclasses
According to recent studies, multiple subpopulations of B cells vary in location, phenotype, and function. Some of these subpopulations represent temporal stages of B-cell development, after leaving the B cell from the bone marrow. Some B cells like B-1a, B-1b, B-2, and marginal zone B cells represent different subpopulations of mature B cells.
The existence of the B-1 B-cell subpopulation was first described in 1983 in the lab of Leonard and Leonore Herzenberg. The researchers discovered a set of B cells bearing the CD5 antigen. This antigen’s expression was thought to be restricted to T-cells. These B cells bearing CD5, are B-1 B cells that make up only 5% of B cells in humans and mice. B-1 B cells appear before B-2 B cells during embryonic development. B-1b B cells are the B cells, which have the functional characteristics of B-1 cells but lack the expression of the CD5 molecules.
B cells secret IL-10 and act as negative regulators
A population of B cells negatively regulates inflammatory immune responses by secreting the cytokine IL-10. The cytokine is normally associated with regulatory T cells. It has pleiotropic effects on other immune system cells. Due to this effect, the suppression of T-cell production of the cytokines IL-2, IL-5, and TNF-α takes place. Interaction of IL-10 with antigen-presenting cells reduces the cell-surface expression of MHC antigens.
B cells, with T cells, might down-regulate the function of other immune system cells. B cells producing IL-10 have been identified among B-1 and B-2 B-cell populations. All B-10 B cells secret a diverse collection of antibodies, with specificity for both foreign and auto-antigens. These cells may function as limiting and controlling inflammation during an immune response.
Conclusion
After activation of B cells, it is necessary to stop the proliferation of B cells. Once the pathogen has been eliminated, most B-cells enter into an apoptotic program. This is also known as negative regulation of B-cell activation.
The BCR (B-cell receptor) of activated mature B cells is associated with CD19/CD21 and CD81. In addition to this, the BCR of mature B cells is also associated with an extra transmembrane molecule, CD22. This molecule on its cytoplasmic domain, bears an immunoreceptor tyrosine-based inhibitory motif (ITIM). ITIMs are inhibitory in function.
CD32, also known as receptor FcγRIIb, and like CD22, it bears a cytoplasmic ITIM domain. It recognizes immune complexes containing IgG antibodies. B-cell activation is hindered by the binding of circulating antigen-IgG complexes to the B-cell surface. CD5 acts as a negative regulator in both BCR and TCR signaling pathways.
A population of B cells negatively regulates inflammatory immune responses by secreting the cytokine IL-10. B cells and T cells might down-regulate the function of other immune system cells. B cells producing IL-10 have been identified among B-1 and B-2 B-cell populations.
You may also like:
- The development of B cells defined by immunoglobulin gene rearrangements
- Factors regulating the development of B cell

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.



