In this article, I briefly describe various immunotherapies for the treatment of cancer. These therapies focus on stimulating the immune system to recognize and attack cancer cells. Immunotherapies work by generating signals or immune cells that direct the body’s defense mechanisms toward effectively targeting and eliminating tumors.
Toward Immunotherapy
There are many therapies adapted for the treatment of cancer. Discrete solid tumors are surgically removed, and local radiation is often employed to destroy residual malignant cells. Adjuvant cancer therapy involves the use of various drugs or small-molecule inhibitors designed to target residual or metastatic tumor cells, while neoadjuvant therapies administer drugs before surgery to reduce tumor size and provide prognostic insight into therapeutic effectiveness. The choice of therapy depends on the type of cancer and the specific characteristics of the tumor cells.
Drug-based treatments for cancer broadly fall into four categories: chemotherapies, hormonal therapies, targeted therapies, and immunotherapies. While these approaches can reduce tumor burden, they often cause significant side effects, face resistance, and may not eliminate metastatic cells.
Immunotherapies are redefining cancer treatment by harnessing the patient’s immune system to recognize and destroy tumor cells. By overcoming immune evasion mechanisms, therapies like immune checkpoint inhibitors, CAR-T cells, and cancer vaccines have achieved durable responses in cancers once considered untreatable.
Mechanism of Cancer Immunotherapies
Immunotherapies are treatments specifically designed to stimulate, restore, or enhance the body’s natural antitumor immune responses. These therapies work by activating the immune system against cancer cells, generating signals or immune cells that guide the body to target and eliminate tumors. Approaches include humanized monoclonal antibodies that selectively bind to cancer cells, tumor-derived peptides delivered to induce cytotoxic T lymphocyte (CTL) activity, or molecules that block tumor-mediated suppression of immune activation. Often, immunotherapy is combined with standard or experimental treatments such as surgery, radiation, or chemotherapy to boost overall immune effectiveness.
The Origins and Evolution of Cancer Immunotherapy
In the late 19th century, cancer surgeon William B. Coley pioneered a groundbreaking approach by injecting bacteria directly into the inoperable tumor of a bone cancer patient. This gives unexpectedly positive results. This led Coley and several other physicians to continue experimenting with bacterial preparations, later termed “Coley’s toxins,” as an early form of cancer immunotherapy for treating aggressive tumors. Although this method was eventually replaced by chemotherapy and radiotherapy, it laid the foundation for modern immune-based treatments.
Today, immunotherapy encompasses four main types, each targeting a distinct component of the immune system. These approaches may involve administering specific antibodies or T cells, thereby enhancing the body’s humoral and cellular immune responses to combat cancer more effectively.
Monoclonal Antibody Therapy
Monoclonal antibodies (mAbs) have been pivotal in the field of cancer immunotherapy, with over a dozen approved for clinical use—most designed to recognize specific surface molecules on cancer cells. A landmark example was demonstrated by Ron Levy and his team at Stanford, who treated a 64-year-old patient suffering from advanced B-cell lymphoma that had spread to the liver, spleen, and bone marrow (Figure 1). Since all cancerous B cells displayed the same membrane-bound immunoglobulin idiotype, the researchers developed a mouse-derived monoclonal antibody specifically targeting this idiotype.
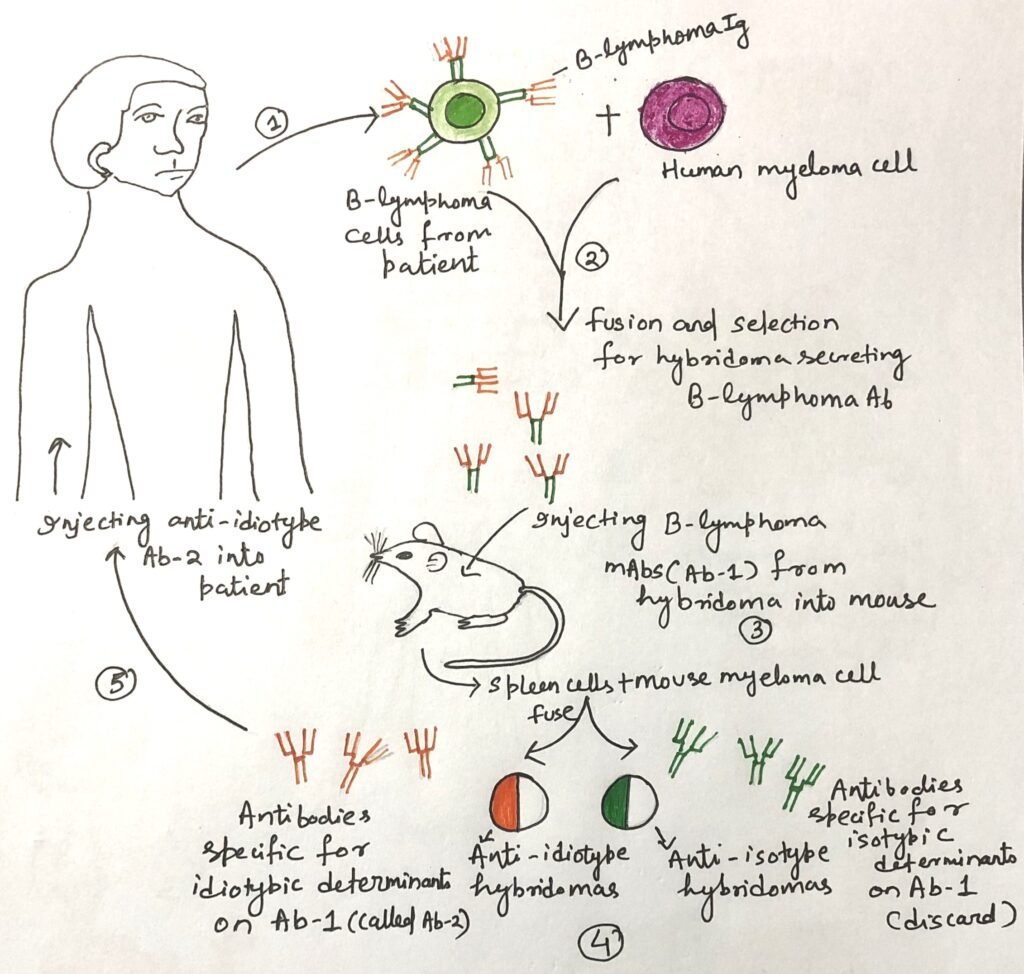
Upon administration, the anti-idiotype antibody bound exclusively to the malignant B cells, triggering their destruction through complement activation or antibody-dependent cellular cytotoxicity (ADCC), while leaving healthy cells unharmed. Remarkably, after just four injections, the patient’s tumors regressed, leading to an extended remission period.
Although producing patient-specific idiotype antibodies proved costly and time-consuming, the same group advanced a broader strategy. They developed therapies targeting common B-cell surface antigens, such as CD20, which is expressed on the majority of B cells. This led to the development of therapeutic monoclonal antibodies (mAbs), such as rituximab, which is now widely used for treating non-Hodgkin lymphoma and other B-cell malignancies.
Antibody-Drug Conjugates: Precision Weapons in Targeted Cancer Therapy
Antibody-drug conjugates (ADCs) represent a cutting-edge form of targeted cancer therapy in which monoclonal antibodies are linked to toxic agents, effectively creating “guided missiles” against cancer cells. These toxic components may include radioactive isotopes, chemotherapeutic drugs, or highly potent toxins, enabling the delivery of lethal doses directly to tumor cells while largely sparing healthy tissues. When the conjugated molecule is specifically a toxin, the compound is referred to as an immunotoxin.
The first FDA-approved ADC, Mylotarg, combined an anti-CD33 antibody with a cytotoxic agent to treat acute myeloid leukemia (AML). However, it was later discontinued due to limited therapeutic success and elevated patient mortality.
Many cancers exhibit overexpression of growth factors or their receptors, making them ideal targets for antibody-based treatments. For instance, approximately 25–30% of metastatic breast cancer cases show amplification of the HER2 (human epidermal growth factor receptor 2) gene, resulting in excessive production of the HER2 protein—a receptor present only in trace levels in normal tissues. This molecular difference paved the way for Herceptin (trastuzumab), a humanized monoclonal antibody that specifically binds to HER2, effectively treating HER2-positive breast cancers.
Building on this success, researchers developed an immunotoxin derivative called Kadcyla (T-DM1). They gained approval to treat certain metastatic HER2-positive breast cancers. Kadcyla couples Herceptin with a cytotoxic agent (DM1) that binds to tubulin, disrupting the cancer cell’s mitotic machinery and thereby halting cell division. This dual-action approach combines targeted recognition with potent intracellular killing—maximizing efficacy while minimizing collateral damage.
Adoptive Cell Transfer
Although tumor-infiltrating lymphocytes (TILs), T cells that naturally migrate into tumors, are present in most solid cancers, they often fail to eliminate malignant cells, indicating a loss of functional activity within the tumor microenvironment. In a pivotal study involving metastatic melanoma patients, researchers extracted TILs and expanded them in vitro using interleukin-2 (IL-2) to reverse their anergic (inactive) state. After administering lymphodepleting treatments to make space for the reintroduced immune cells, patients received infusions of these activated autologous TILs.
The results were notable: nearly 50% of patients experienced significant tumor regression, and around 10% achieved long-term or complete remission. However, responses varied—many patients showed minimal or no improvement. This discrepancy was likely due to the presence of regulatory T cells (TREGs) in non-responding individuals. These immunosuppressive cells, which express a high-affinity IL-2 receptor, can outcompete effector T cells for IL-2, thus dampening immune activation.
To overcome these limitations, current cell-based immunotherapies increasingly employ peripheral blood-derived T cells rather than TILs. This strategy, known as adoptive cell transfer (ACT), helps minimize the expansion of suppressor cells and enhances the efficacy of tumor-targeted immune responses.
CAR T Cells: Engineering the Immune System to Fight Cancer
Chimeric Antigen Receptor (CAR) T-cell therapy represents a revolutionary advancement in adoptive T-cell immunotherapy for cancer. The process begins by isolating T cells from the patient’s own blood (autologous T cells), followed by genetic modification in vitro to introduce a synthetic receptor gene that enables these cells to specifically recognize tumor antigens. Once scientists engineer the CAR T cells, they expand and reinfuse them into the patient to seek out and destroy cancer cells.
In 1989, Gideon Gross and his team at the Weizmann Institute of Science (Israel) laid the foundation for this approach. He designed the first artificial T-cell receptor, a fusion of antibody-derived antigen-binding domains and T-cell signaling components. This chimeric structure combined the precise target recognition ability of B-cell receptors with the potent cytotoxic action of T cells. The resulting CAR T cells marked a major milestone in cancer immunotherapy, providing a powerful and precise tool to reprogram the immune system against malignancies.
Therapeutic Cancer Vaccines
Scientists design prophylactic vaccines to stimulate immunity before infection and prevent disease. However, therapeutic vaccines enhance or modify existing immune responses after infection or antigen exposure. Both approaches fall under the broad category of immunotherapy. The active ingredient in such vaccines, known as an immunogen, is a protein or antigen that elicits an immune reaction. However, immunogens that are highly effective in preventive vaccines often fail in therapeutic contexts, since the immune system has already encountered the antigen. For example, the human papillomavirus (HPV) vaccine prevents infection by cancer-causing HPV strains with up to 99% efficacy, yet it remains ineffective in women already infected with the virus.
Early studies on cancer vaccines in mice explored methods to enhance the presentation of tumor-specific antigens. In one experiment, researchers cultured mouse dendritic cells (DCs) with granulocyte-macrophage colony-stimulating factor (GM-CSF) and exposed them to tumor fragments before reintroducing them into the mice. These dendritic cells then activated T helper (TH) cells and cytotoxic T lymphocytes (CTLs) specific to the tumor antigens, which enabled the mice to resist tumor growth when later challenged with live cancer cells.
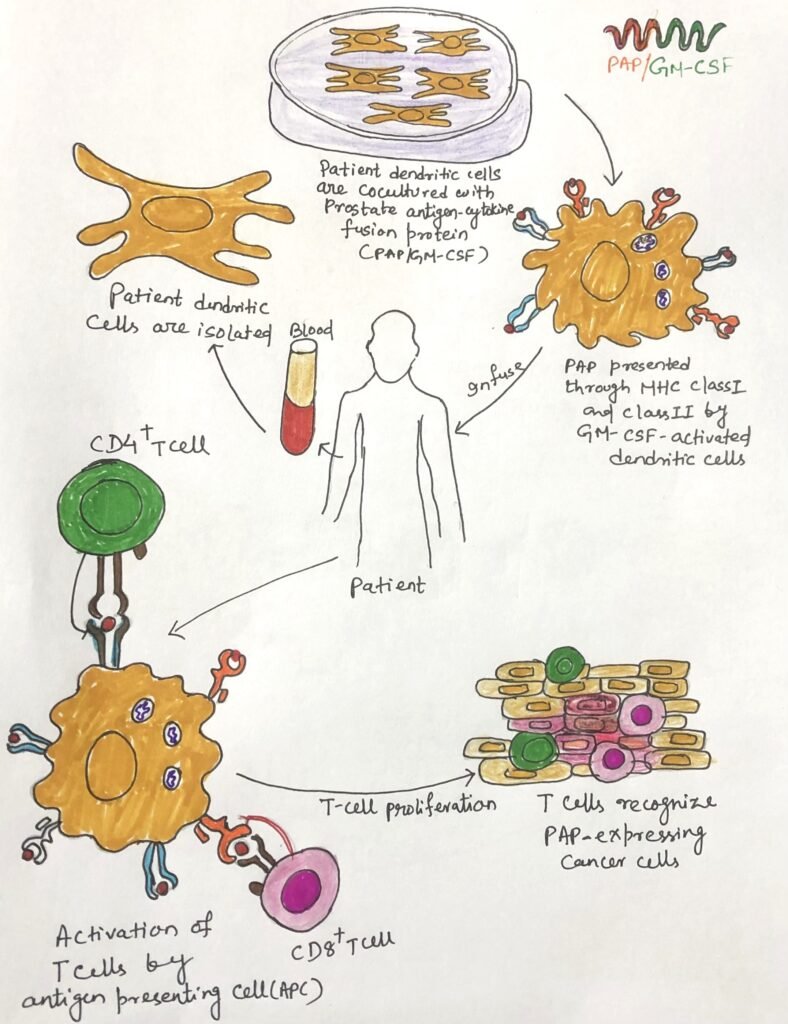
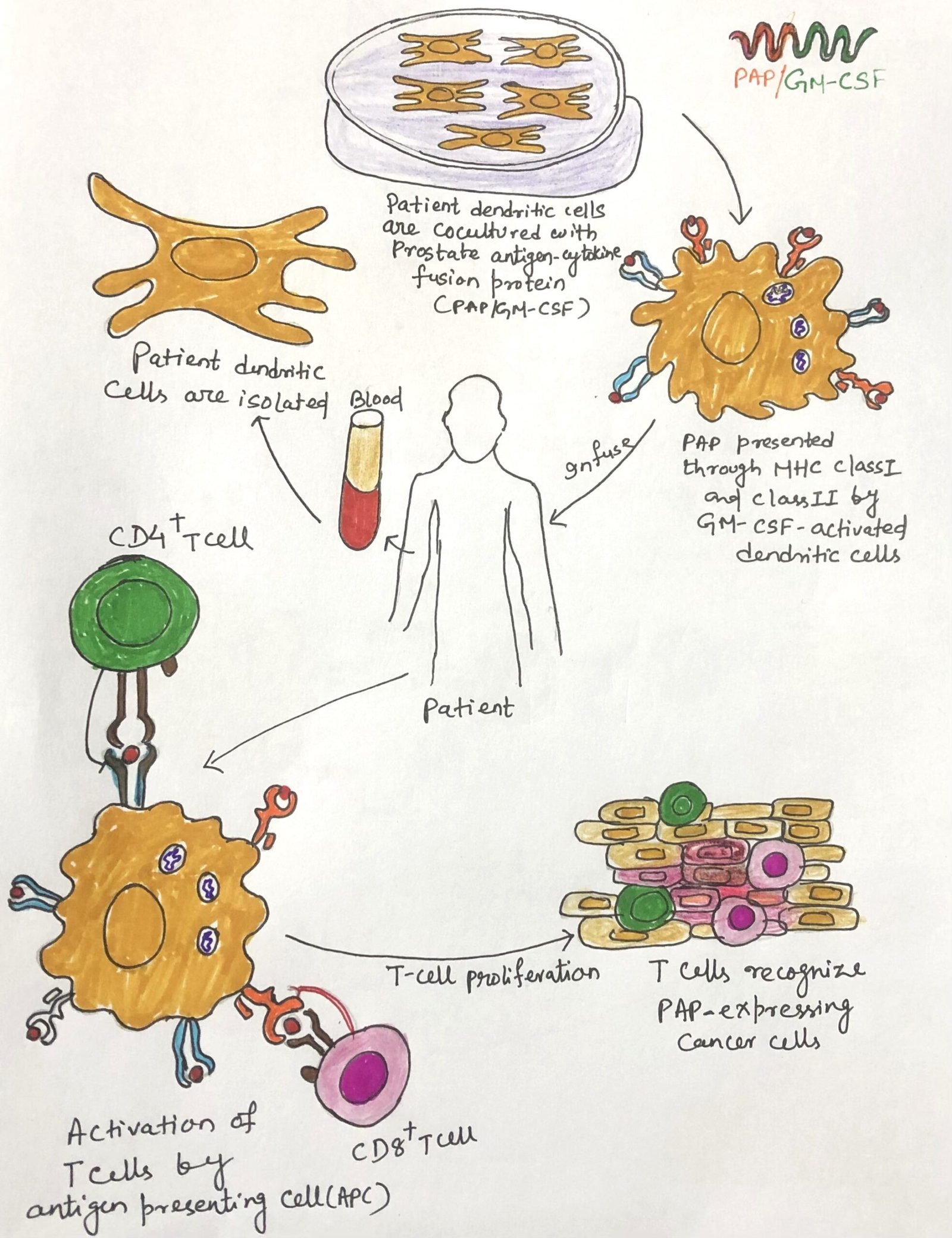
Building upon this concept, researchers developed Sipuleucel-T, the first FDA-approved therapeutic cancer vaccine, designed to treat prostate cancer. In this treatment, scientists isolate autologous dendritic cells from the patient and culture them in vitro with a fusion protein that combines prostatic acid phosphatase (PAP), a prostate tumor antigen, with GM-CSF. They then infuse the activated antigen-presenting cells (APCs) back into the patient, where these cells initiate an immune response that specifically targets prostate cancer cells.
Emerging Cancer Vaccines
Despite early optimism, therapeutic cancer vaccines have delivered limited success over the past decade, making them one of the more disappointing immunotherapy approaches. However, newer innovations are offering renewed promise. One such advancement is T-VEC (Talimogene laherparepvec), a genetically engineered oncolytic herpesvirus modified to include the gene for granulocyte-macrophage colony-stimulating factor (GM-CSF). When injected directly into melanoma lesions, T-VEC selectively infects and lyses tumor cells, releasing tumor antigens and stimulating an immune response against remaining cancer cells.
Beyond viral-based therapies, researchers are exploring neoantigen-based cancer vaccines, which target unique tumor-specific epitopes arising from DNA mutations that generate nonself proteins. These neoantigens are not present in normal tissues and are distinct for each patient’s tumor. So, they represent precise and personalized therapeutic targets. This emerging approach seeks to unmask hidden tumor antigens and train the immune system to recognize and eliminate malignant cells with unprecedented precision.
Checkpoint Inhibitors Unlock the Immune System’s Power Against Cancer
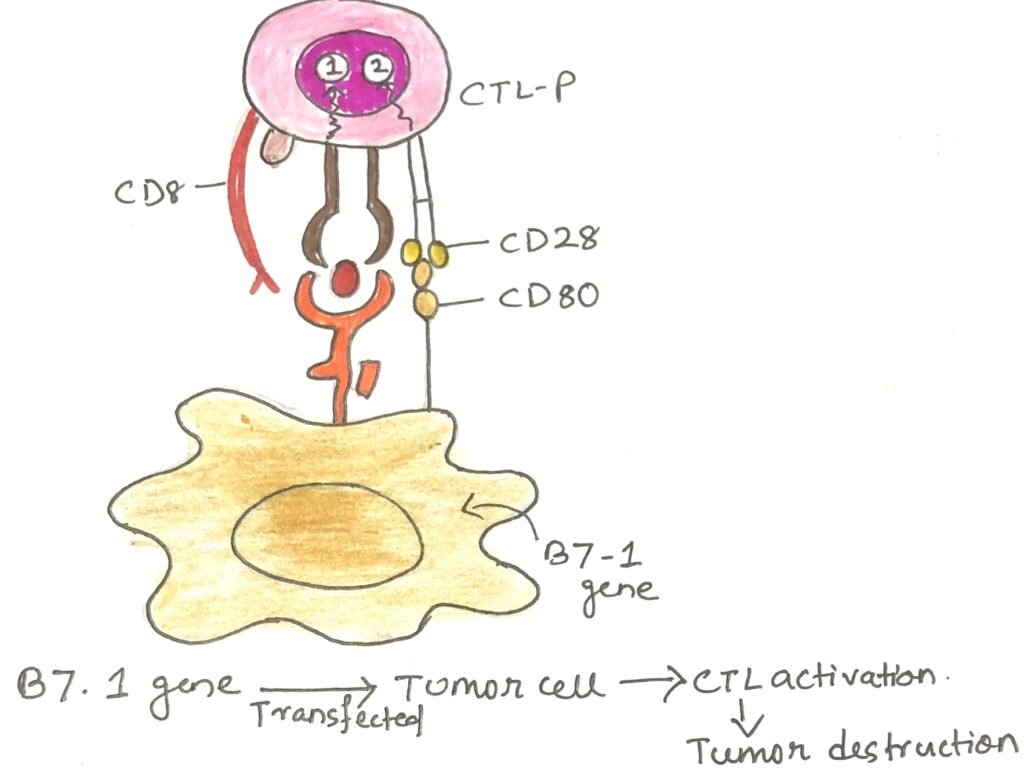
The tumor microenvironment in aggressive cancers is often unfavorable for immune activation. This makes it difficult for T cells to attack cancer cells effectively. One key reason is that cancer cells lack co-stimulatory signals, which are essential for complete T-cell activation. When a T cell receives only the first signal, T-cell receptor (TCR) engagement—without the second signal provided by CD28 binding to CD80/86 on professional antigen-presenting cells (pAPCs)- it becomes anergic, or inactive. This led scientists to hypothesize that enhancing co-stimulation might help the immune system recognize and destroy cancer cells.
In 1992, Peter Linsley and his team supported this idea by showing that 40% of mice with tumors experienced complete regression when injected with melanoma cells engineered to express CD80/86. The following year, Sarah Townsend and James Allison took this further by vaccinating mice with irradiated, CD80-transfected melanoma cells (Figure 3). When researchers later exposed the mice to malignant melanoma cells lacking CD80, nearly 90% of them resisted the cancer, demonstrating the power of co-stimulation in anti-tumor immunity.
However, the immune system also uses co-inhibitory molecules, known as checkpoints, to regulate its activity and prevent overreaction. Allison and his colleagues discovered that blocking one such checkpoint protein, CTLA-4, using a monoclonal antibody (mAb), could trigger tumor rejection even in animals with established cancers. After extensive clinical testing, the FDA approved ipilimumab in 2011, marking the arrival of a new generation of checkpoint inhibitor drugs that work by releasing the immune system’s natural brakes to help it fight cancer.
PD-1 Blockade: A New Era in Immune-Based Cancer Therapy
The programmed death-1 (PD-1) receptor on T cells functions similarly to CTLA-4, acting as a brake on immune activation. Rather than competing for co-stimulatory signals, PD-1 actively binds to its ligand PD-L1, which dendritic cells normally express but tumor cells often express abnormally(Figure 4). This interaction suppresses T-cell activity, allowing cancers, especially those with poor clinical outcomes, to evade immune destruction.
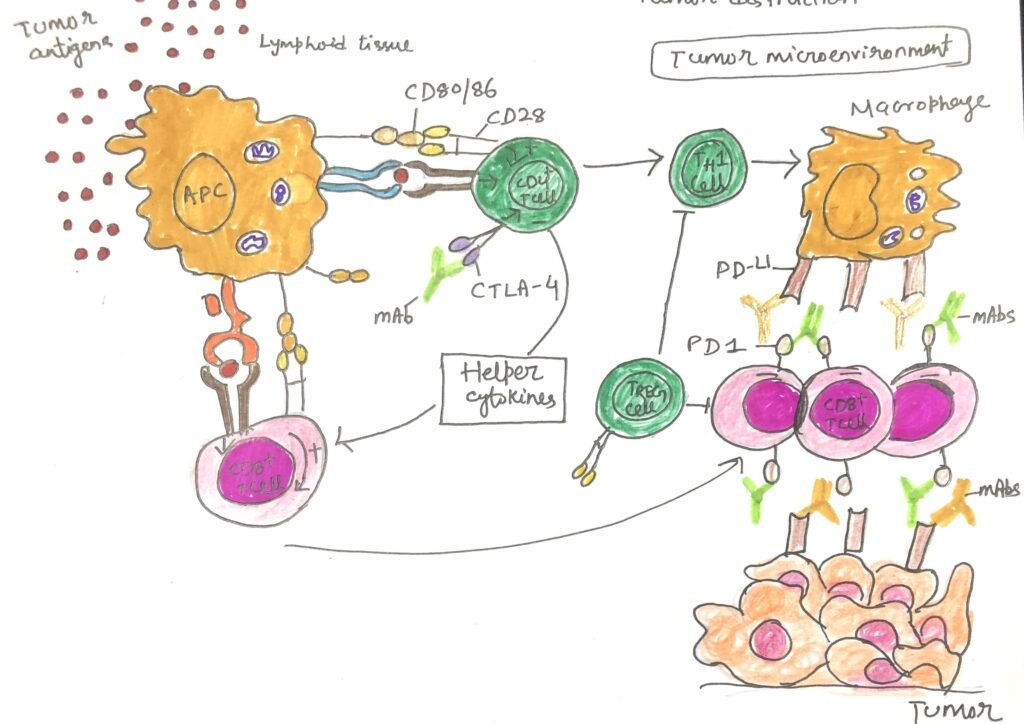
While immune checkpoint inhibitors that block PD-1 or PD-L1 have revolutionized cancer therapy, they can also cause widespread immune dysregulation. As a result, patients may experience immune-related adverse effects, including inflammation or autoimmune attacks affecting the skin, gastrointestinal tract, liver, and endocrine glands.
Despite these side effects, checkpoint blockade therapy represents a breakthrough in oncology, underscoring the central role of the immune system in combating cancer. This approach has not only improved survival rates in several cancers but also transformed the way scientists and clinicians view cancer treatment. It shifts the focus toward harnessing and guiding the body’s own immune power.
Conclusion
The evolution of cancer immunotherapy represents one of the most significant achievements in modern medicine. From Coley’s early experiments with bacterial toxins to today’s advanced strategies, such as monoclonal antibodies, antibody-drug conjugates, adoptive cell transfer, CAR T-cell therapy, therapeutic vaccines, and immune checkpoint inhibitors, each breakthrough has deepened our understanding of how the immune system can be mobilized to fight cancer.
While challenges remain, such as treatment resistance, side effects, and the high cost of personalized therapies, these approaches have already transformed outcomes for many patients once deemed untreatable. The success of checkpoint inhibitors and CAR T cells, in particular, highlights the immune system’s remarkable potential when properly directed.
Overall, cancer immunotherapy has shifted the paradigm from simply destroying cancer cells to empowering the body’s own defenses to recognize, attack, and remember malignant cells. Ongoing research and innovation will deliver more effective and targeted treatments and offer a future in which scientists can manage or even cure cancer using the power of immunity.
You may also like:
- Tumor Immune Escape Mechanisms and Apoptotic Resistance
- Subunit vaccines– vaccines from purified macromolecules
- Improvement in Vaccine Immunogenicity and Enhancing Immune Response
- Viruses as Hidden Causes of Cancer

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.