In this article, I provide a brief description of G proteins, which play crucial roles in various signaling processes. However, defects in G proteins lead to the development of many diseases.
G Proteins
Guanine nucleotide-binding proteins (G proteins) are a group of proteins that function as molecular switches within cells. They help transmit signals from various external stimuli to the cell’s interior. Their function is regulated by mechanisms that influence their capacity to bind and hydrolyze guanosine triphosphate (GTP) into guanosine diphosphate (GDP). In the GTP-bound state, they are considered “on,” while in the GDP-bound state, they are “off.” These proteins are part of the broader family of enzymes known as GTPases.
G proteins are categorized into two main types. The first type operates as monomeric small GTPases (small G-proteins), while the second type functions as heterotrimeric G protein complexes. These heterotrimeric complexes consist of three subunits: alpha (Gα), beta (Gβ), and gamma (Gγ). Moreover, the beta and gamma subunits can associate to form a stable dimer, known as the beta-gamma complex.
G Proteins: Molecular Switches with Built-In Timers
G proteins play diverse roles across a wide range of cellular activities. The groundbreaking work of Alfred G. Gilman and Martin Rodbell revealed the involvement of guanosine nucleotide-binding proteins (G proteins) in processes such as sensory perception, regulation of cell division, growth and differentiation, intracellular transport of proteins and membrane vesicles, and protein synthesis.
The human genome encodes nearly 200 different G proteins, which vary in size, subunit composition, cellular location, and specific function. Despite these differences, all G proteins share a defining trait—they can switch between active and inactive states. After activation, they automatically inactivate themselves within a short time, functioning as molecular binary switches equipped with built-in timing mechanisms.
The superfamily of G proteins
The G protein superfamily encompasses various members, including trimeric G proteins involved in adrenergic signaling (GS and Gi) and vision (transducin), as well as small G proteins with diverse roles. Examples include Ras, which participates in insulin signaling; ARF and Rab, which regulate vesicle trafficking; Ran, which controls transport into and out of the nucleus; Rho, which governs cell cycle timing; and several proteins essential for protein synthesis, such as initiation factor IF2 and elongation factors EF-Tu and EF-G. Many G proteins carry covalently attached lipid groups, providing membrane affinity and determining their specific cellular localization.
Molecular Switch Mechanism of G Proteins
All G proteins share a conserved core structure and operate through a common mechanism that switches between two states: an inactive conformation and an active conformation. The binding of GDP leads to an inactive conformation, whereas GTP binding creates an active conformation. The Ras protein, a minimal signaling unit, serves as the prototype for understanding this mechanism.
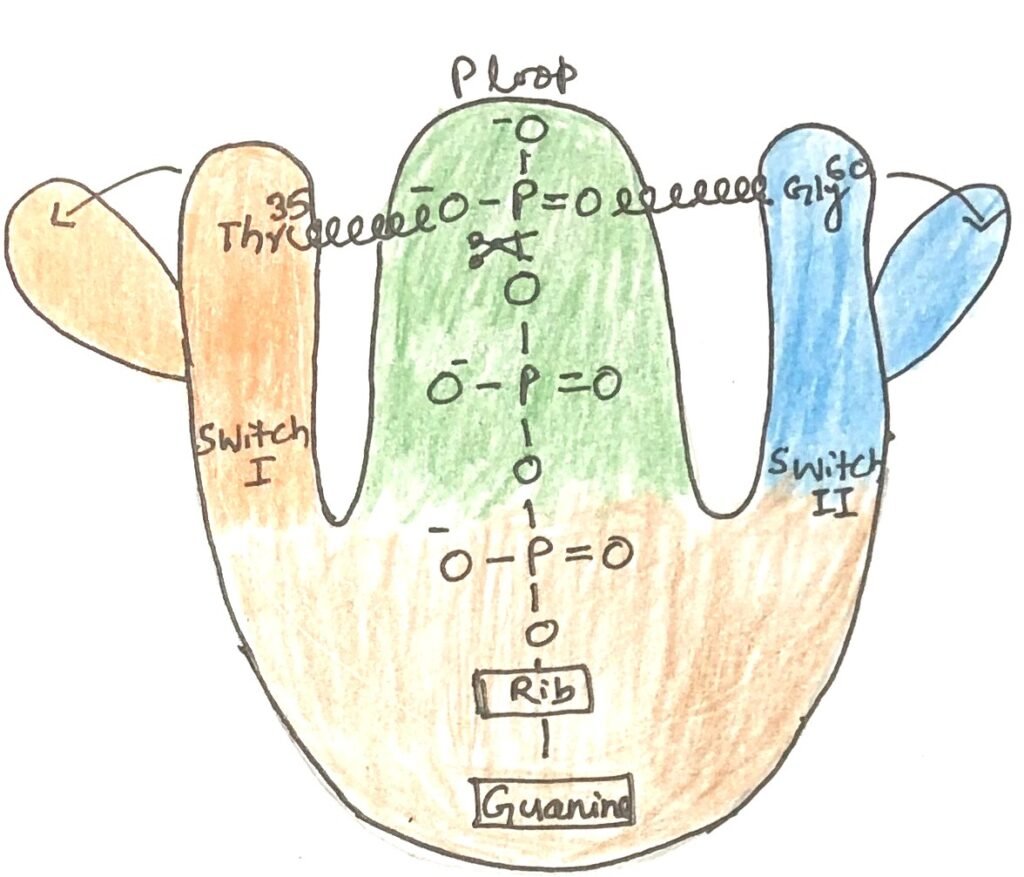
In the GTP-bound state, Ras and other G proteins undergo a conformational change that exposes two previously hidden regions, switch I and switch II, which interact with downstream signaling partners. This active state persists until the G protein hydrolyzes its bound GTP to GDP, thereby inactivating itself.
The key determinant of this conformational change is the γ-phosphate of GTP. In Ras, the γ-phosphate binds to a Lys residue in the phosphate-binding loop (P loop) and two critical residues: Thr35 in switch I and Gly60 in switch II (Figure 1). These residues form hydrogen bonds with the γ-phosphate oxygens, acting like springs that hold the protein in its active shape. In the figure, Rib is the ribose moiety of guanosine. The ribose sugar is covalently linked to guanine.
When GTP is cleaved to GDP and inorganic phosphate (Pi) is released, these stabilizing hydrogen bonds are lost. The protein then relaxes into its inactive conformation, concealing the sites that, in the active form, mediate interactions with other molecules.
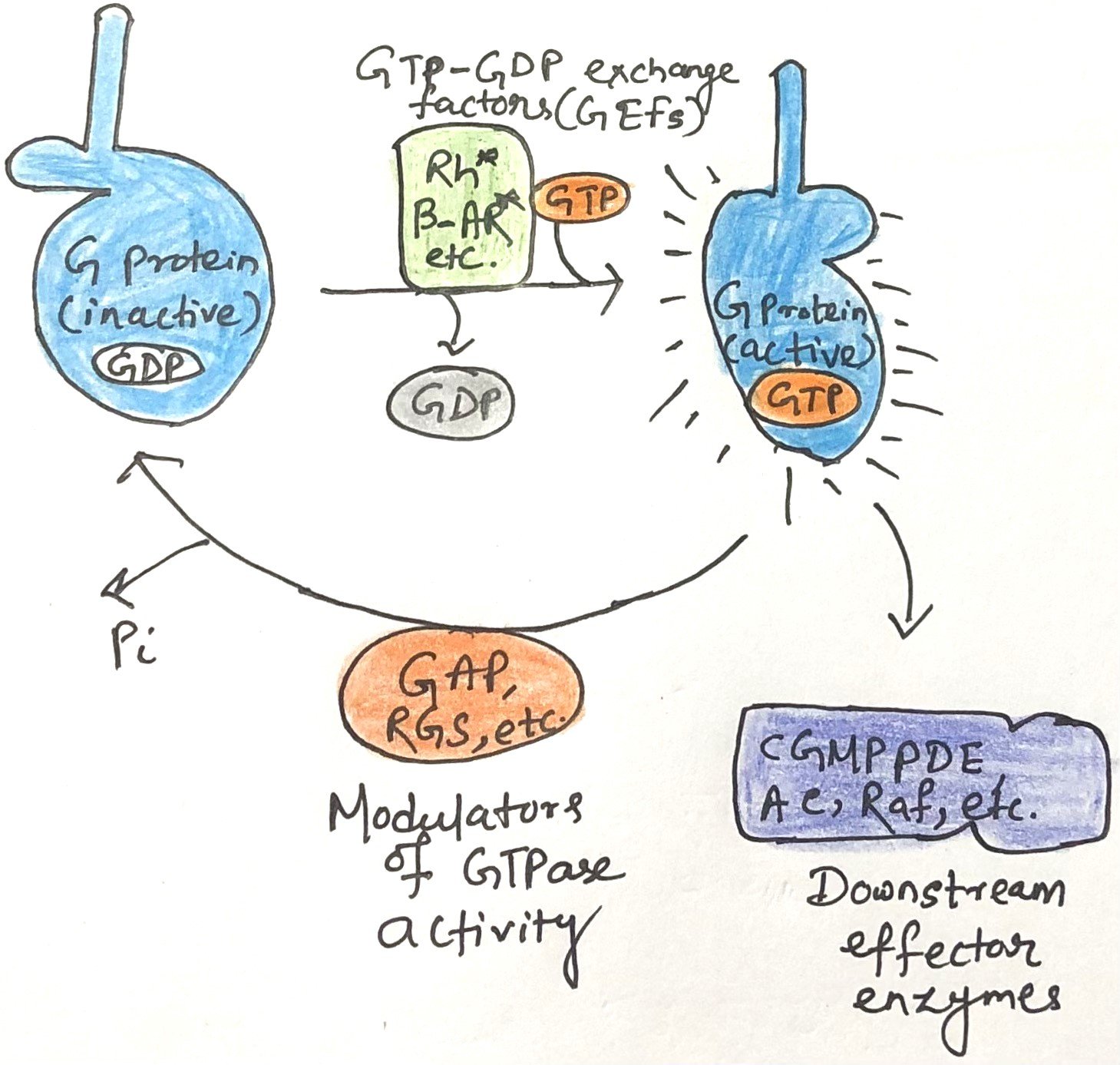
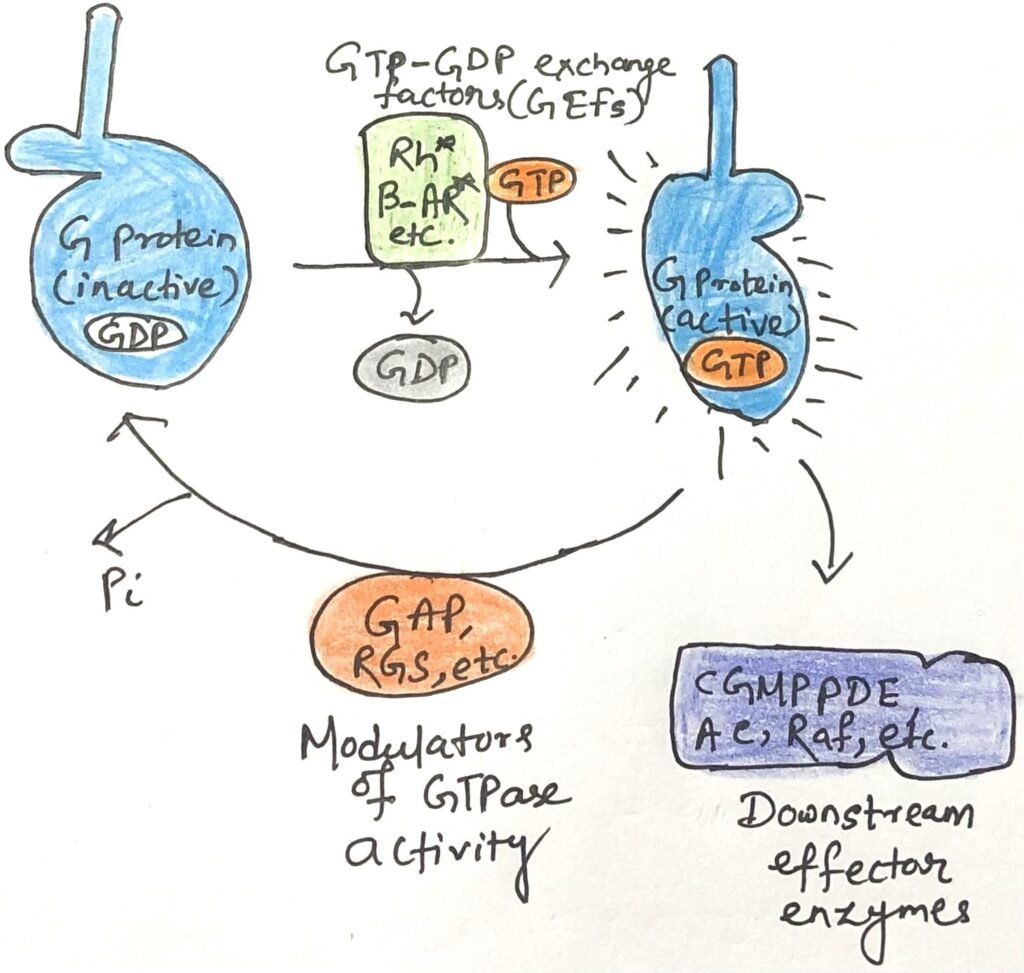
Factors Regulating the Activity of G Proteins
The activity of G proteins is tightly controlled by multiple regulatory factors. GTPase-activating proteins (GAPs) accelerate the intrinsic GTPase activity of most G proteins by as much as 10⁵-fold. In the case of heterotrimeric G proteins, these GAPs are also known as regulators of G protein signaling (RGSs). Both GAPs and RGSs influence how long the molecular switch remains in the “on” state by contributing a key arginine residue to the GTPase active site, thereby promoting catalysis.
Switching a G protein on requires the exchange of bound GDP for GTP—a naturally slow process. This is greatly facilitated by guanine nucleotide-exchange factors (GEFs), which associate with G proteins and promote GDP release (Figure 2). The ligand-bound β-adrenergic receptor is one example of a GEF, while many other proteins can serve as GAPs.
Together, the opposing actions of GEFs (activating) and GAPs/RGSs (inactivating) determine the concentration of GTP-bound G proteins, thereby modulating both the intensity and duration of cellular responses to incoming signals.
G Protein Malfunctions and Their Implications for Health
G proteins are central players in numerous signaling pathways. However, defects in their function can lead to serious diseases. Notably, mutations in Ras proteins are implicated in roughly 25% of all human cancers. These mutations often occur in key residues near the GTP-binding site or within the phosphate-binding loop (P loop), effectively abolishing Ras’s GTPase activity.
Under normal conditions, Ras becomes active upon binding GTP and is later inactivated through GTP hydrolysis. However, when its GTPase function is lost, Ras remains constitutively active, driving continuous cell division—even in cells that should remain quiescent.
The NF1 gene, a tumor suppressor, encodes a GAP that normally accelerates Ras’s GTPase activity, ensuring timely inactivation. Mutations in NF1 that disable this GAP leave Ras reliant solely on its intrinsically slow GTPase activity. As a result, once Ras binds GTP, it stays active for prolonged periods, persistently sending the “divide” signal to the cell, contributing to uncontrolled proliferation.
Impact of G Protein Subunit Mutations on Cellular Signaling and Disease
The α subunit of the stimulatory G protein (Gαs) regulates intracellular cAMP levels in response to hormonal signals. Mutations in the Gαs-encoding gene can produce subunits that are either permanently active or permanently inactive. Activating mutations, often occurring in residues essential for GTPase activity, cause persistent cAMP elevation, triggering abnormal downstream effects such as excessive cell proliferation. These mutations are present in approximately 40% of pituitary tumors. In contrast, inactivating mutations render cells unresponsive to hormones that signal via cAMP.
Mutations in other G protein subunits are also linked to disease. For example, a defect in the α subunit of transducin (Tα), a key player in visual signaling, disrupts its interaction with rod outer segment phosphodiesterase, leading to a form of night blindness. Additionally, a common genetic variant in the β subunit of heterotrimeric G proteins is associated with hypertension and may contribute to obesity and atherosclerosis.
Mechanism of Cholera Toxin Action on Host G Proteins
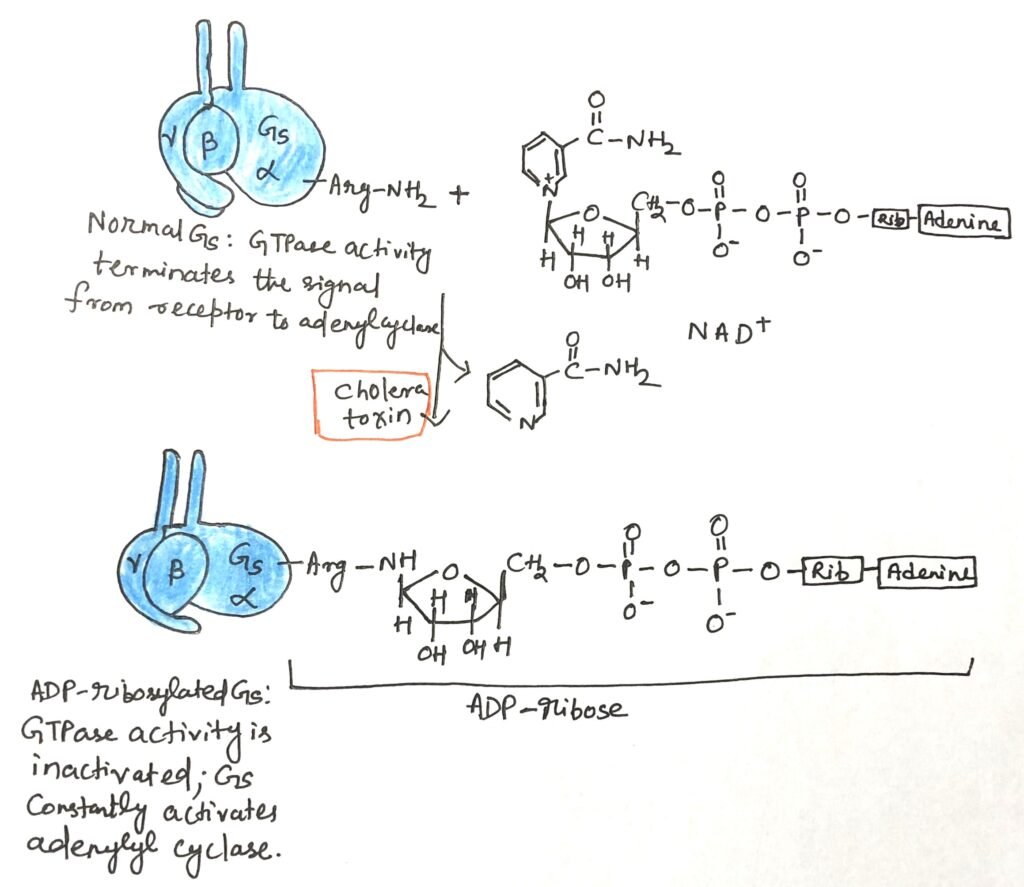
The pathogenic bacterium Vibrio cholerae, which causes cholera, produces a toxin in the intestine that disrupts normal G protein–mediated signaling in host cells. This toxin is a heterotrimeric protein composed of subunit B and subunit A. Subunit B binds specifically to gangliosides on the surface of intestinal epithelial cells, enabling the entry of subunit A into the cell. Once inside, subunit A is cleaved into two fragments, A1 and A2. The A1 fragment interacts with the host cell’s ADP-ribosylation factor ARF6—a small G protein—by binding to residues in its switch I and switch II regions, which are exposed only when ARF6 is in its active state.
This association activates A1, enabling it to transfer an ADP-ribose group from NAD⁺ to a critical arginine residue in the P loop of the Gαs subunit (Figure 3). ADP-ribosylation inhibits Gαs GTPase activity, locking it in its active form. Consequently, adenylyl cyclase remains persistently stimulated, leading to chronically elevated cAMP levels. The sustained cAMP activates protein kinase A (PKA), which phosphorylates the CFTR chloride channel and the Na⁺/H⁺ exchanger. This results in massive NaCl efflux into the intestinal lumen, creating an osmotic imbalance that draws large amounts of water into the gut. The outcome is profuse watery diarrhea, rapid dehydration, and severe electrolyte loss. Prompt oral or intravenous rehydration is essential to prevent death.
Conclusion
Guanine nucleotide-binding proteins (G proteins), are a group of proteins that function as molecular switches within cells. They help transmit signals from various external stimuli to the cell’s interior. The human genome encodes close to 200 different G proteins. These vary in size, subunit composition, cellular location, and specific function. G proteins play diverse roles across a wide range of cellular activities.
All G proteins share a conserved core structure and operate through a common mechanism that switches between two states: an inactive conformation and an active conformation. The binding of GDP leads to an inactive conformation, whereas GTP binding creates an active conformation. In the GTP-bound state, Ras and other G proteins undergo a conformational change that exposes two previously hidden regions, switch I and switch II, which interact with downstream signaling partners.
The activity of G proteins is tightly controlled by multiple regulatory factors. G proteins are central players in numerous signaling pathways, but defects in their function can lead to serious diseases. Notably, mutations in Ras proteins are implicated in roughly 25% of all human cancers. Mutations in other G protein subunits are also linked to disease. For example, a defect in the α subunit of transducin (Tα), a key player in visual signaling. It disrupts its interaction with rod outer segment phosphodiesterase, leading to a form of night blindness.
The pathogenic bacterium Vibrio cholerae causes cholera. It produces a toxin in the intestine that disrupts normal G protein–mediated signaling in host cells.
You may also like:
- Role of G Protein-Coupled Receptors in Vertebrate Olfaction and Gustation
- β-Adrenergic Receptor-Mediated Signal Transduction Involving Adenylyl Cyclase, cAMP, and PKA

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.