In this article, I briefly describe costimulatory receptors CD28 and ICOS and how they bind with their ligands.
Costimulatory receptors
Co-stimulatory signals are required for optimal T-cell activation. T-cell non-responsiveness arises from high affinity TCR-MHC interactions in the absence of functional antigen presenting cells (APCs). It is called T cell anergy. The interaction between specific co-stimulatory receptors on T cells and co-stimulatory ligands expressed by only professional antigen presenting cells, generate signal 2. A T cell after getting both signal 1 and 2, produces cytokines that enhances its entry into the cell cycle and proliferation.
CD28 is the most common co-stimulatory receptors expressed by professional antigen presenting cells. ICOS is the co-stimulatory receptor expressed by B cells, some APCs, and T cells.
CD28- The first costimulatory molecule
In 1989, Navy immunologist Carl June described T cell proliferation and activation by introducing the costimulatory molecule CD28. Recently, it had been identified as a dimeric glycoprotein expressed on all human CD4+ T cells and half of the CD8+ T cells. It co-operates with T-cell receptor signals, thus inducing expression of the pro-proliferative cytokine IL-2. In this way, it enhances TCR-induced proliferation and survival.
How CD28 was discovered
Carl June and his colleagues isolated T cells from human blood by the process of density gradient centrifugation. They depleted a T-cell enriched population of cells not expressing CD28. Then, they measured the response of CD28+ T cells to stimulation of T cell receptor (TCR), in the presence or absence of CD28 engagement (figure 1).
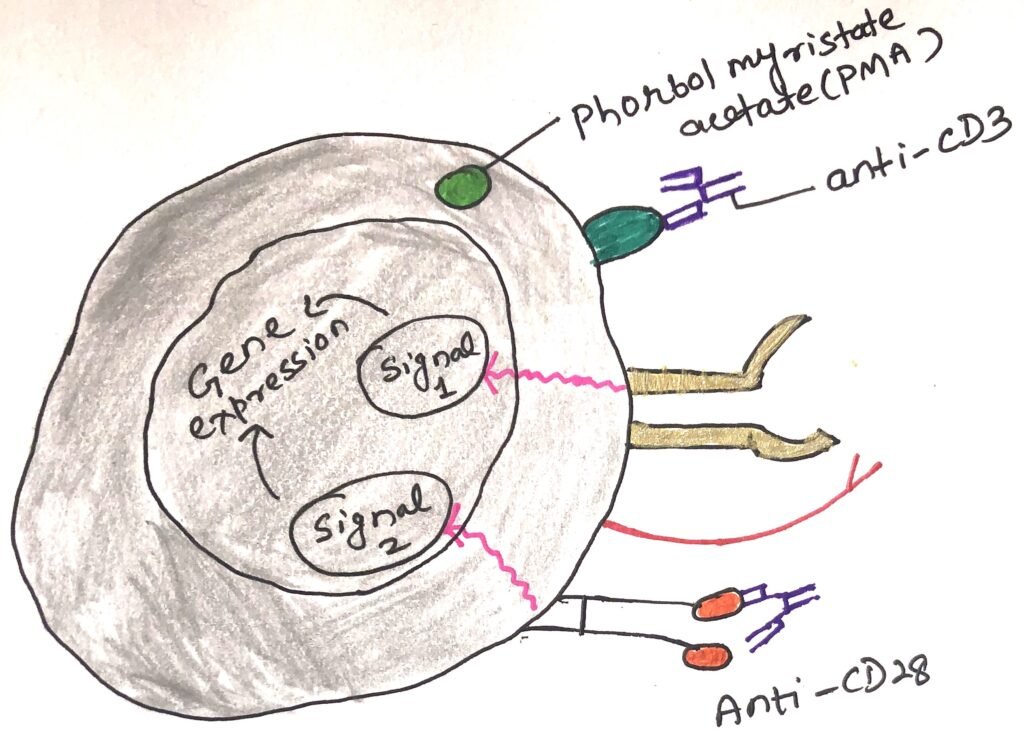
June and colleagues used either monoclonal antibodies to the CD3 complex or PMA (phorbol myristate acetate), a protein kinase C to mimic the TCR-MHC interaction. They used an anti-CD28 monoclonal antibody to engage the CD28 molecule (figure 1). They added two negative controls, one of which included a population that was cultured in a growth medium with no additives. The second population was exposed to phytohemagglutinin (PHA), which activate T cells only in the presence of APCs.
Measurement of the proliferation of each population
They measured the proliferation of each of these populations. They did it by measuring incorporation of a radioactive (tritiated) nucleotide, uridine, incorporated by cells that are synthesizing new RNA, thus showing signs of activation. When responses particularly to PMA were examined, it showed striking results (figure 2). According to the expectations, T cells grown in the absence of stimulation or half stimulation, remained quiescent. Cells treated with stimuli (causing T cell proliferation), were activated and showed evidence of activation with CD3 engagement.
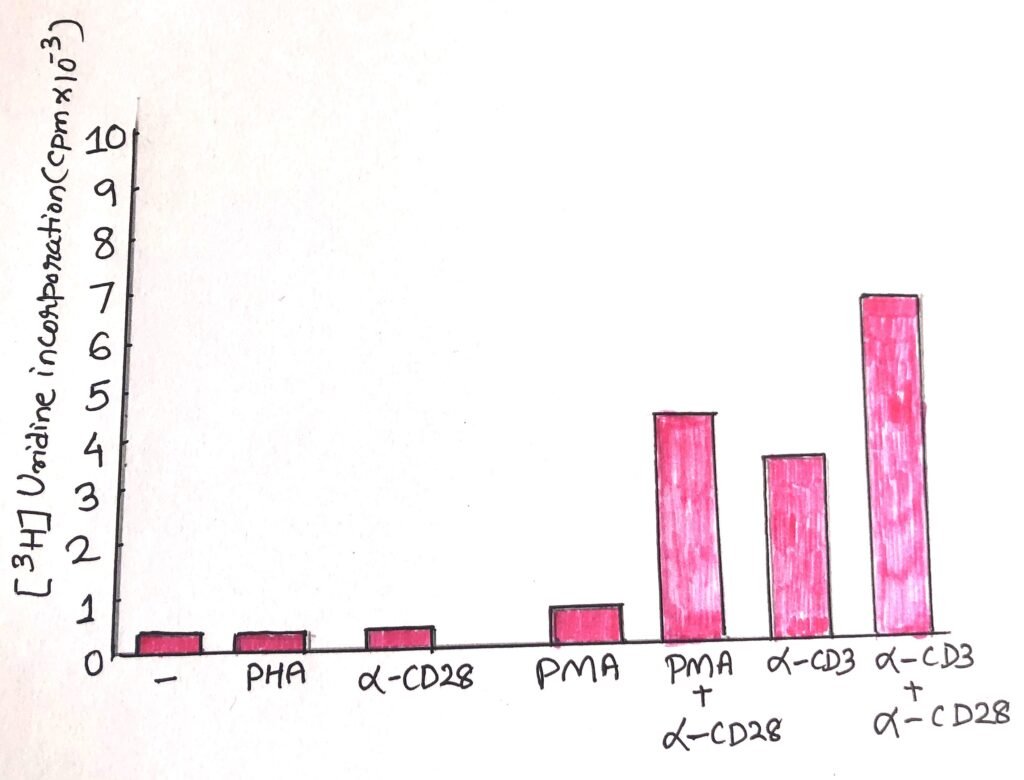
The cells treated with anti-CD28 only remained quiscent as the negative control samples. This indicated that engagement with only CD28 could not induce activation. However, when CD28 was engaged along with exposed to PMA, it showed marked rise in incorporation of uridine. When T cells were treated with both anti-CD28 and anti-CD3 also took up more uridine than the T cells treated with only anti-CD3.
June and colleagues used northern blot analysis and functional assays to characterize the cytokines in culture supernatant of these stimulated cells. They showed that CD28 stimulation induced anti-CD3-stimulated T cells to produce more cytokines involved in antiviral, anti-tumor and proliferative activity, producing an enhanced T-cell immune response.
During the time of the publication of this article, the researchers were unaware of the natural ligand for CD28. Now it is known that CD28 binds to CD80/86 and provides the critical signal 2 during the activation of naïve T-cell.
Binding of CD28 to its ligands
CD28 has two ligands from the B7 family of proteins, i.e., CD80 (B7-1) and CD86 (B7-2). These members of the immunoglobulin superfamily have similar extracellular domains. However, they vary in their intracellular domains, which shows that they may not only act as ligands but may generate signals influencing antigen presenting cells.
Most of the T cells in our body express CD28 co-stimulatory molecule. However, most of them do not express its ligands. Only professional antigen presenting cells can express CD80/86. The best activator of naive T cells are the mature dendritic cells, which constitutively express Cd80/86. Macrophages and B cells when activated by a pathogen, they up-regulate the expression of CD80/86.
CD28 signaling makes both qualitative and quantitative contributions to enhance the strength of TCR signaling. CD28 recruits PI3 kinase, which activates other downstream kinases that in turn regulate many aspects of cell metabolism, survival and division.
ICOS- The costimulatory receptor
ICOS, the inducible co-stimulator gives positive co-stimulation for T-cell activation. However, ICOS does not bind with CD80 and CD86, rather it binds with another member of the B7 family, ICOS ligand (ICOS-L). A subset of activated antigen presenting cells express ICOS ligand.
CD28 and ICOS positive costimulatory receptors play distinct roles in T-cell activation. Naïve T cells express CD28 but do not express ICOS. Memory T cells and effector T cells express ICOS. CD28 plays a key co-stimulatory role at the time of activation initiation. ICOS plays a vital role in maintaining the activity of effector and memory T cells after their differentiation.
Conclusion
CD28 is the most common co-stimulatory receptors expressed by professional antigen presenting cells. ICOS is the co-stimulatory receptor expressed by B cells, some APCs, and T cells.
Carl June and his colleagues measured the response of CD28+ T cells to stimulation of T cell receptor (TCR), in the presence or absence of CD28 engagement. June and colleagues used either monoclonal antibodies to the CD3 complex or PMA (phorbol myristate acetate). PMA is a protein kinase C, which mimics the TCR-MHC interaction. They showed that CD28 stimulation induced anti-CD3-stimulated T cells to produce more cytokines involved in antiviral, anti-tumor and proliferative activity, producing an enhanced T-cell immune response.
The costimulatory receptor CD28 has two ligands from the B7 family of proteins, i.e., CD80 (B7-1) and CD86 (B7-2). CD28 signaling makes both qualitative and quantitative contributions to enhance the strength of TCR signaling.
ICOS, the inducible co-stimulator does not bind with CD80 and CD86, rather it binds with another member of the B7 family, ICOS ligand (ICOS-L). A subset of activated antigen presenting cells express ICOS ligand. CD28 and ICOS positive costimulatory receptors play distinct roles in T-cell activation. ICOS plays a vital role in maintaining the activity of effector and memory T cells after their differentiation.
You may also like:
- The effector cells of the immune system
- The coinhibitory receptors: CTLA-4, PD-1, and BTLA
- The absence of a costimulatory signal leads to clonal anergy

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.



