In this article, I briefly describe the conventional imaging techniques based on immunofluorescence. These techniques use fluorescently labeled antibodies to visualize specific proteins or other biomolecules within cells and tissues. These methods provide valuable insights into cellular structure, localization of molecules, and biological processes under a microscope.
Fluorescence-Based Antibody Imaging Techniques
Fluorescence microscopy allows precise visualization of specific molecules within cells and tissues by using antibodies that bind to target antigens. These antibodies are coupled to fluorescent dyes, which emit light of a characteristic wavelength when excited, making the antigen–antibody interaction directly observable. This method forms the basis of immunofluorescence, which enables the mapping of proteins, organelles, or signaling molecules within cells. It also underlies techniques such as flow cytometry, which utilizes fluorescence to detect and analyze different cell populations in suspension.
Depending on the approach, labeling may be carried out through direct immunofluorescence (fluorescent dye attached to the primary antibody) or indirect immunofluorescence(a secondary dye-labeled antibody binding the primary antibody), the latter often providing stronger signals. Owing to its high specificity, sensitivity, and versatility, fluorescence-based antibody imaging has become an essential tool for both fundamental research and clinical diagnostics, with applications ranging from studying disease mechanisms to identifying pathogens.
Fluorescent Dyes and Their Specificity in Biomolecular Detection
Some molecules can absorb light at one wavelength and then emit it at a longer wavelength, a process known as fluorescence. When this emitted light falls within the visible spectrum, fluorescent dyes become powerful tools for detecting molecules to which they are attached.
Many fluorescent dyes show selective binding to certain biological macromolecules. For instance, the blue dye DAPI (4’,6-diamidino-2-phenylindole) binds strongly to DNA, allowing visualization of nuclei. Phalloidin is widely used to stain filamentous actin, while annexin A5 specifically recognizes phosphatidylserine exposed on apoptotic cells, making fluorescent annexin A5 a valuable marker for detecting cell death.
In addition, immunofluorescence techniques employ antibodies or proteins such as streptavidin that can be conjugated with fluorescent dyes to tag a broad range of targets. By integrating these diverse labeling approaches, researchers can obtain highly detailed images that reveal both the overall organization and fine structures within cells.
Use of Natural Fluorescent Proteins in Imaging
Along with synthetic dyes, certain naturally occurring proteins, such as GFP (green fluorescent protein) and RFP (red fluorescent protein), possess built-in fluorescent chromophores. In many experiments, scientists place the genes for these proteins under the control of specific promoters. They may create fusion proteins that link GFP to a native protein. These strategies enable fluorescence imaging to track when and where proteins regulated by those promoters are expressed. This provides powerful insights into gene expression and protein localization.
Fluorescence Microscopy for Visualizing Molecules
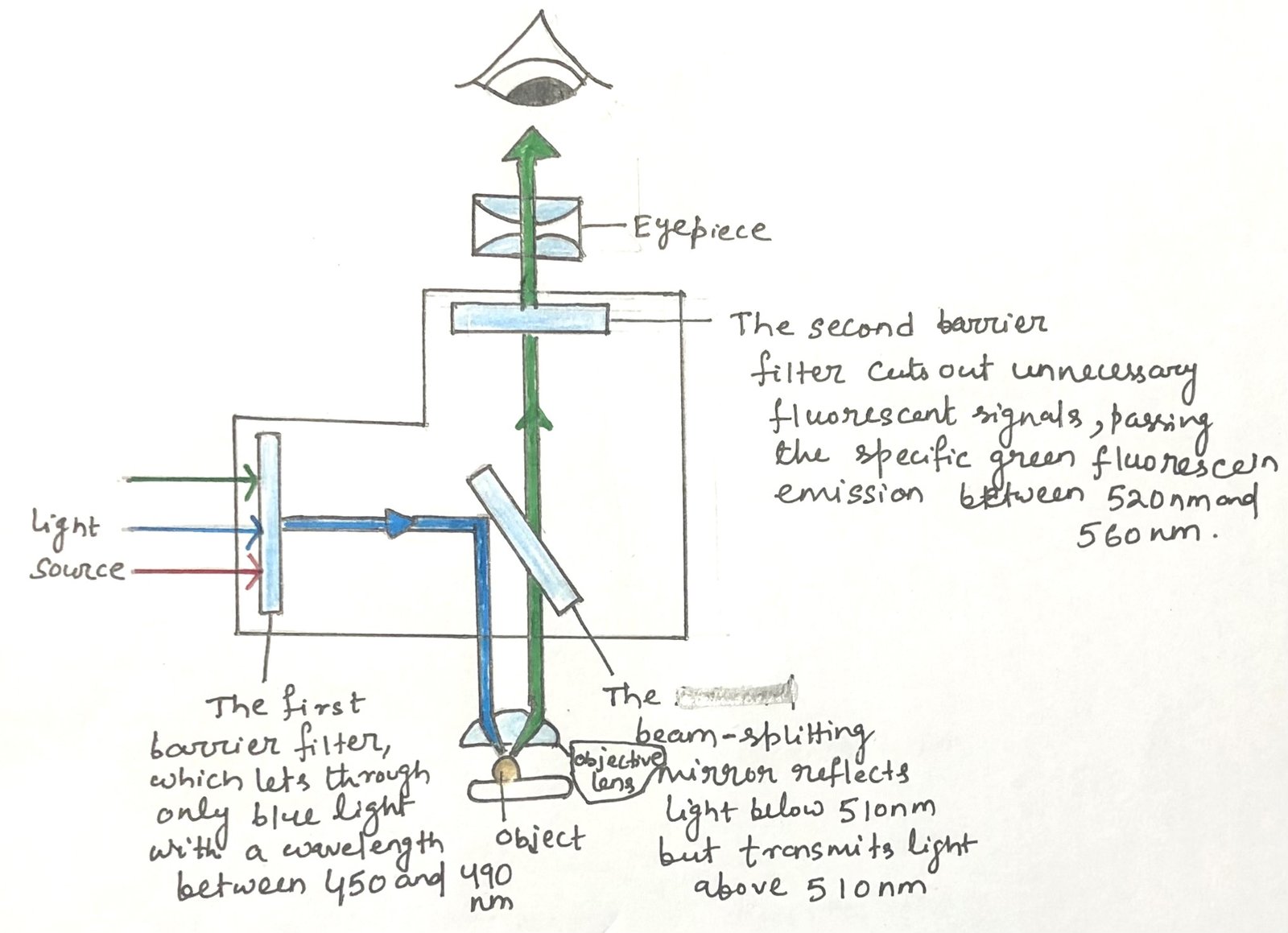
Fluorescence microscopy works by using short-wavelength light to excite fluorescent dyes bound to molecules in cells or tissues. The microscope is equipped with a series of filters and mirrors that control which wavelengths of light are allowed to pass through. Thus, the researcher can choose which fluorescent signals to observe (Figure 1).
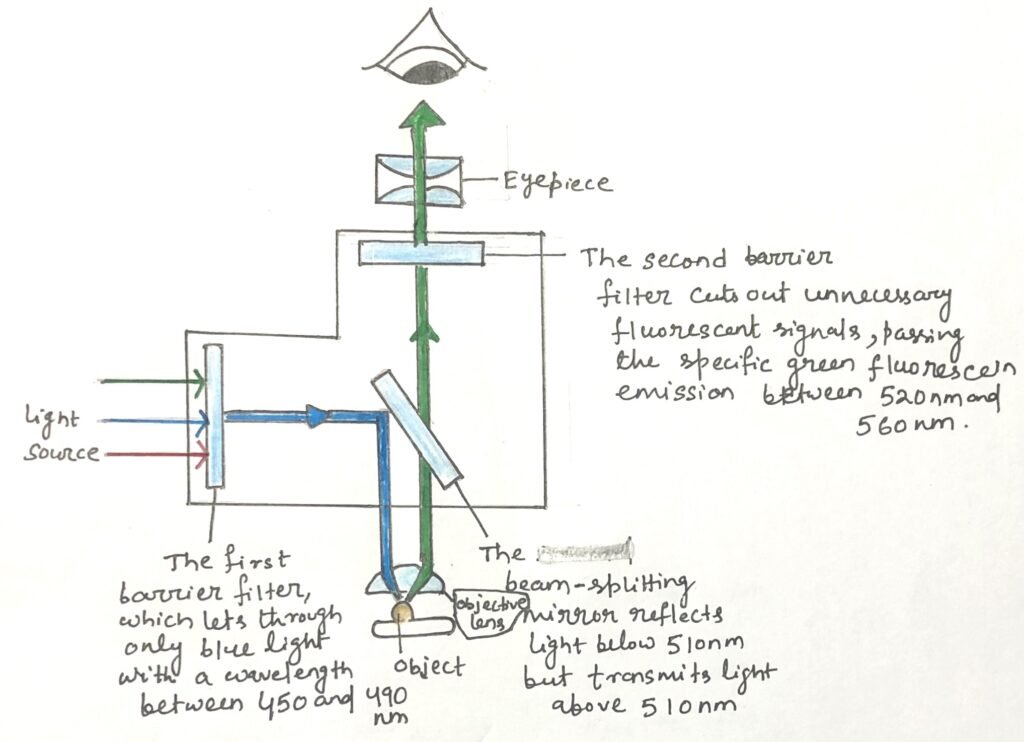
Modern fluorescence microscopes often use multiple filters and mirrors. Thus, it can detect emissions from several different dyes at the same time. The images produced in different colors can then be digitally overlaid by the microscope’s software, resulting in a single composite image. This combined image allows researchers to clearly compare the locations and interactions of various antibody-bound molecules within the same sample.
High-Resolution Three-Dimensional Imaging with Confocal Fluorescence Microscopy
A limitation of conventional fluorescence microscopy is that molecules above and below the focal plane also emit light. The light reaches the objective and produces blurred images. In Figure 2, a laser beam illuminates the sample and excites fluorescent dyes located at multiple focal planes, shown here as the orange, blue, and purple dotted lines.
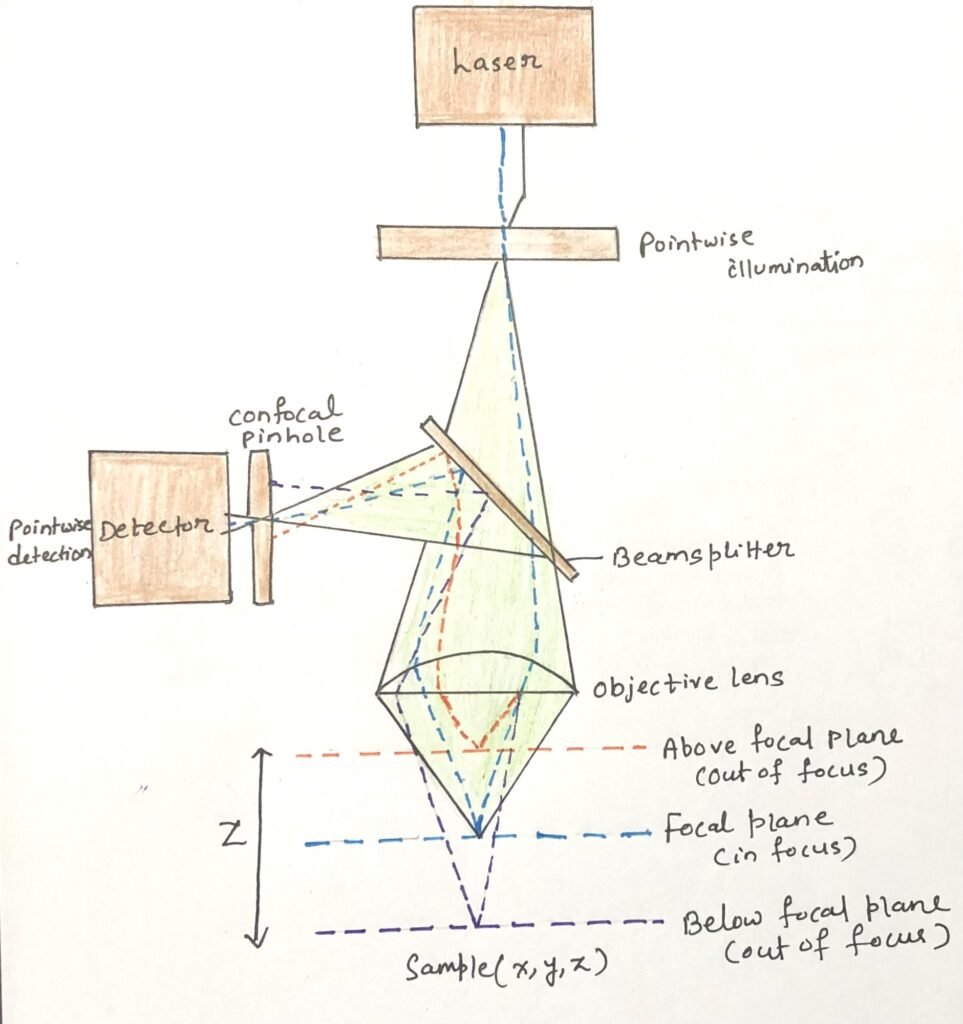
Confocal microscopy overcomes this problem by directing light from the focal plane through a pinhole aperture (Figure 2) placed in front of the detector. Light emitted from out-of-focus regions is blocked at the edges of the pinhole. This allows the capture of sharp images from a single optical section.
In laser scanning confocal microscopy, lasers are used as the light source. The computer control shifts the focal plane across three dimensions. By scanning the sample in the x and y axes at different depths, the instrument reconstructs detailed and precise three-dimensional images of the specimen.
Limitations of Standard Confocal Microscopy
In conventional confocal microscopy, the laser excites fluorescent probes along its entire path through the tissue. As a result, even though the detected emission originates from a single focal plane, probes at multiple levels of the sample are also activated. Over time, this widespread excitation leads to photobleaching, where probes lose their ability to fluoresce, thereby reducing the sample’s longevity. Additionally, the unwanted light generated from out-of-focus regions must be filtered out before producing the final image.
Multiphoton Fluorescence Microscopy
This technique employs long-wavelength lasers that emit light in the infrared region. These beams are relatively low in energy. Thus, excitation of fluorescent molecules requires the simultaneous absorption of two or more photons to provide the necessary energy. The use of infrared lasers reduces photobleaching and extends the lifespan of the sample, since areas exposed to only a single beam are generally unaffected.
Importantly, fluorescence excitation occurs only at the precise plane where multiple laser beams intersect, ensuring high specificity. By shifting the focal point across the x and y axes, a complete optical section can be captured. The repetition of this process at successive z-levels enables the reconstruction of a detailed three-dimensional image.
Conclusion
Fluorescence microscopy enables accurate visualization of molecules in cells and tissues through antibodies that specifically recognize target antigens. By attaching fluorescent dyes to these antibodies, the bound antigens can be detected as the dyes emit light at defined wavelengths upon excitation.
Fluorescence is the process by which certain molecules absorb light at one wavelength and emit it at a longer wavelength. When this emission falls in the visible range, fluorescent dyes become powerful tools for molecular detection. Some dyes bind selectively to specific macromolecules. DAPI labels DNA in cell nuclei, phalloidin stains filamentous actin, and annexin A5 detects phosphatidylserine on apoptotic cells, serving as a marker of cell death.
Fluorescence microscopy works by using short-wavelength light to excite fluorescent dyes bound to molecules in cells or tissues. Modern fluorescence microscopes often use multiple filters and mirrors. Thus, it can detect emissions from several different dyes at the same time.
Confocal microscopy directs light from the focal plane through a pinhole aperture placed in front of a detector. Light emitted from out-of-focus regions is blocked at the edges of the pinhole. It allows the capture of sharp images from a single optical section.
Multiphoton fluorescence microscopy uses long-wavelength infrared lasers. Here, excitation of fluorescent molecules occurs through the simultaneous absorption of two or more low-energy photons. This approach minimizes photobleaching and prolongs sample viability, as regions exposed to only a single beam remain largely undamaged.
You may also like:
- The antigen-antibody interaction- Immunofluorescence
- Quantitative Cell Analysis Using Flow Cytometry

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.