In this article, I briefly describe the common diseases associated with the intestine. Inflammatory bowel disease, Crohn’s disease, and Celiac disease are common intestinal diseases.
Our Intestinal Immune Response and Diseases
The immune system’s response to both commensal and pathogenic microbes is not without cost. Even when inflammation successfully eliminates pathogens, it can still damage the delicate epithelium, which must then rely on the continuous work of stem cells to repair and regenerate. When a pathogen resists clearance, however, inflammation can turn chronic, reshaping the intestinal structure and altering the composition of its cellular and immune landscape, leaving lasting imprints on the body’s defenses.
Inflammatory Bowel Disease (IBD)
It is among the most prevalent chronic conditions affecting the intestines. It encompasses two distinct disorders—Crohn’s disease and ulcerative colitis—both marked by persistent inflammation of the gut lining. This inflammation leads to symptoms like pain and diarrhea.
Crohn’s disease is a type of IBD that can impact any section of the digestive tract. It is commonly linked to an abnormal type 1 immune response and is marked by scattered clusters of inflammatory macrophages within the mucosa. These clusters can develop into granulomas, which are hard to eliminate and disrupt the small intestine’s microenvironment (Figure 1).

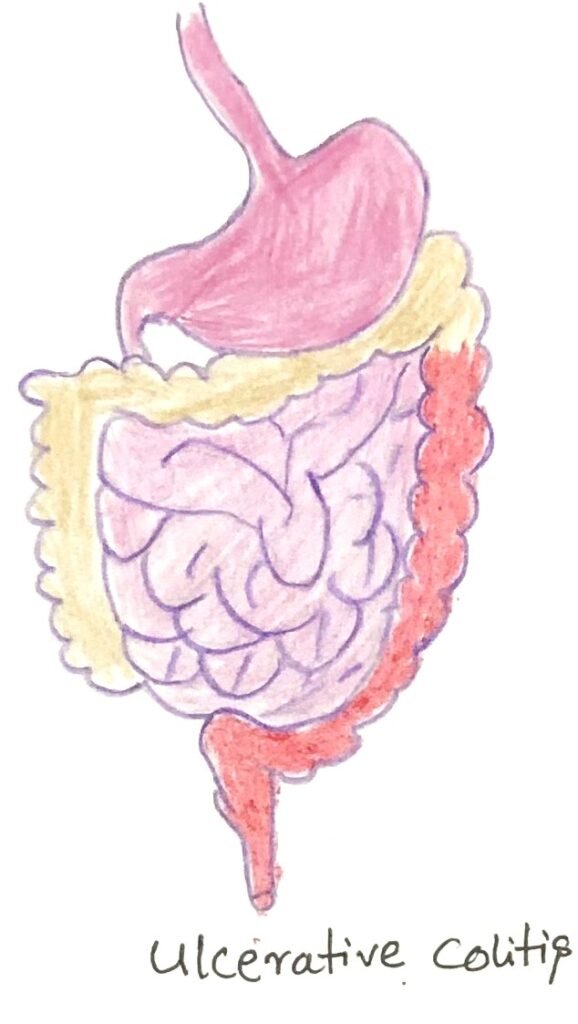
Ulcerative colitis is a type of IBD that specifically affects the descending portion of the large intestine (figure 2). It is generally linked to an abnormal type 2 immune response, leading to active inflammation of the mucosa. This inflammation is characterized by neutrophil infiltration, weakened mucus barriers, and direct injury to the epithelial layer.
Causes of IBD
The reasons for IBD vary from person to person. The causes can be genetic, environmental, or infectious. Decreased mucus production, antimicrobial peptide secretion, maintenance of tight junctions, regulatory T cell generation, and IL-10 levels have all been linked to inflammatory bowel disease (IBD). Loss of tolerance can result if alterations in any of these activities happen. The makeup of the commensal gut microbiome, along with its interaction with mucosal immunity, plays a crucial role in the onset, persistence, and treatment of inflammatory bowel disease (IBD). The disruption of a healthy microbiome, known as dysbiosis, is now a well-accepted contributing factor to IBD. The increasing rates of IBD in industrialized countries point to diet’s impact on the microbiome as a fundamental cause.
Gene Variations Play a Role in the Development of IBD
According to genome-wide association studies, several gene variations are linked with increased susceptibility to IBD. Certain genetic variations suggest defects in pattern recognition receptors, leading to impaired interactions between the gut microbiome and the innate immune system. Multiple innate immune cells in the intestine express NOD2, which is a member of the NLR family of cytosolic pattern recognition receptors. NOD2 recognizes intracellular bacterial MAMPs and activates inflammatory signaling pathways that generate antimicrobial peptides and enhance the immune activity of antigen-presenting cells. People who inherit particular variants of the NOD2 gene may have a risk of up to forty times higher for developing IBD.
CARD9 plays a role in the signaling cascade triggered by certain pattern recognition receptors. A defect in CARD9 indirectly affects the gut microbiome’s composition. This leads to an overall decrease in intestinal tryptophan production. Tryptophan metabolites interact with receptors on the gut epithelium, promoting the release of the protective cytokine IL-22. Since our cells cannot produce tryptophan, it must come from food and the microbiome, and its levels can help partially alleviate symptoms caused by CARD9 mutations.
Extra-Intestinal Manifestations of IBD
Persons suffering from inflammatory bowel disease often suffer from inflammatory disorders of other tissues. Oral ulcers and canker sores are some of the common and painful inflammatory disorders. These extra-intestinal manifestations probably arise from a systemic defect in immune tolerance. The commensal intestinal microbiome helps generate anti-inflammatory cells, including regulatory T cells. These cells populate other organs and tissues other than the intestinal mucosa and contribute to a systemic tolerizing effect. Dysbiosis hinders the development of tolerizing influences and activates proinflammatory TH17 cells. The activated TH17 cell subsets make their way to other organs and cause destruction.
Celiac Disease
It is an autoimmune disorder that affects our intestinal mucosa. Some similar symptoms of the disease with IBD include diarrhea, gastrointestinal discomfort, and bloating. The cause of celiac disease differs from IBD. It is an autoimmune response to dietary gluten and other wheat-associated molecules. These wheat or some other grain-related molecules have penetrated the epithelial barrier. The immune response to gluten leads to the generation of IL-15, which activates IELs (intestinal intraepithelial lymphocytes). This eventually leads to epithelial cell death and physical damage to the barrier. It is characterized by a damaging TH1 response as well as NK-cell and B-cell activity.
People suffering from celiac disease have a different microbiome than healthy individuals. There are different treatment methods for IBD and celiac disease. Patients suffering from celiac disease are advised to be on a gluten-free diet for a lifetime. However, this may not work for some individuals. Doctors also give patients immunosuppressive drugs and antibody-based immunotherapy that blocks inflammatory molecules. This treatment is also not suitable for all.
Conclusion
When inflammation successfully eliminates pathogens, immune responses can still damage the delicate epithelium, which must then rely on the continuous work of stem cells to repair and regenerate. Inflammatory bowel disease is among the most prevalent chronic conditions affecting the intestines. It encompasses two distinct disorders—Crohn’s disease and ulcerative colitis—both marked by persistent inflammation of the gut lining. This inflammation leads to symptoms like pain and diarrhea.
Reduced mucus production, lower antimicrobial peptide secretion, weakened tight junction maintenance, and decreased generation of regulatory T cells and IL-10 all contribute to inflammatory bowel disease (IBD).. According to genome-wide association studies, several gene variations are linked with increased susceptibility to IBD. Certain genetic variations suggest defects in pattern recognition receptors, leading to impaired interactions between the gut microbiome and the innate immune system.
Celiac disease is an autoimmune disorder that affects our intestinal mucosa. Some similar symptoms of the disease with IBD include diarrhea, gastrointestinal discomfort, and bloating. The cause of celiac disease differs from IBD. It is an autoimmune response to dietary gluten and other wheat-associated molecules.
You may also like:
- The intestinal immune system recognizes and responds to pathogens
- Intestinal immunity can initiate both type 1 and type 2 immune responses
- Small and Large Intestines Possess Different Immune Systems
- Normal Flora of the Intestinal Tract

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.




