In this article, I briefly describe some bacterial exotoxins and their pathogenic effects.
Bacterial Exotoxins
Some microorganisms produce poisonous substances called toxins, which play a key role in disease causation. The potency of these toxins can be measured in several ways, most commonly using LD₅₀ (lethal dose 50), the amount that kills 50% of test animals, and MLD (minimum lethal dose), the smallest dose that kills all test animals. Based on their origin and properties, bacterial toxins are classified into two main types: exotoxins and endotoxins.
Among these, exotoxins are the most potent and specific, acting through distinct mechanisms to damage host tissues or interfere with normal cellular functions.
Potency of Exotoxins
Exotoxins are poisonous proteins secreted by living microorganisms. They are extremely powerful, with even trace amounts capable of causing death in animals. Among them, Clostridium botulinum type A produces the most potent toxin known, while Clostridium tetani also generates a highly powerful toxin. The exceptional toxicity of these two is due to their specific action on the mammalian nervous system. Most other exotoxins target different tissues and are comparatively less potent. Some are not fatal but can still cause discomfort, for instance, certain strains of Staphylococcus aureus release a toxin that, even in amounts as small as 1 µg, can trigger severe nausea and vomiting, leading to staphylococcal food poisoning.
Classification of Exotoxins
Exotoxins can be classified according to the tissues or cells they target. For example, botulinum and tetanus toxins specifically affect nervous tissue and are called neurotoxins. V. cholera produces a toxin that targets the intestinal tract and is referred to as an enterotoxin. Diphtheria toxin damages multiple cell types and is therefore classified as a cytotoxin. Some cytotoxins selectively destroy white blood cells and are known as leukocidins, while others lyse red blood cells and are termed hemolysins.
Exotoxins as Toxoids
Exotoxins are proteins in nature and can be rendered non-toxic by treatment with formaldehyde, while still retaining their antigenic properties. In this harmless form, they are known as toxoids, which can stimulate the host’s body to produce antitoxins. This property is vital for protecting susceptible individuals from diseases caused by toxin-producing bacteria. Consequently, toxoids are widely used in vaccines, especially for immunization against tetanus and diphtheria.
Genetic Basis of Exotoxin Production
The ability of bacteria to produce exotoxins is often determined by specific genes, which may be located on the chromosome or carried on mobile genetic elements. In some cases, such as diphtheria and certain forms of botulism, a bacterium gains toxigenic ability through lysogenic conversion, a process in which a temperate bacteriophage introduces the toxin gene into the bacterial genome. Bacteria may also become toxigenic by acquiring plasmids that encode toxin production. For instance, certain Escherichia coli strains cause diarrhea due to a plasmid carrying the gene for an enterotoxin, while some Staphylococcus aureus strains produce enterotoxins responsible for food poisoning through a similar plasmid-mediated mechanism.
Mechanisms of Action of Exotoxins
The process of infection initiates with the adherence of microorganisms to tissue cells. Similarly, an initial adherence of exotoxins to tissue cells is an important first step in their toxic activity. The function of attachment is usually assumed by a particular region or subunit of the toxin. Other regions or subunits of the toxin may have enzymatic activity that causes damage to the tissue cell.
Diphtheria Toxin
The diphtheria toxin is a single polypeptide chain composed of two distinct functional regions, designated as A and B. A and B are linked not only by a peptide linkage but by a disulfide (-S-S-) bridge. The B region enables the toxin to bind to the membrane of a susceptible host cell, after which the A region is cleaved and enters the cell (Figure 1). Inside, the A fragment exhibits enzymatic activity. It catalyzes a reaction that inactivates elongation factor 2 (EF-2). The EF-2 is a crucial protein involved in ribosomal elongation during protein synthesis. Consequently, the affected cell loses its ability to produce proteins and eventually dies.
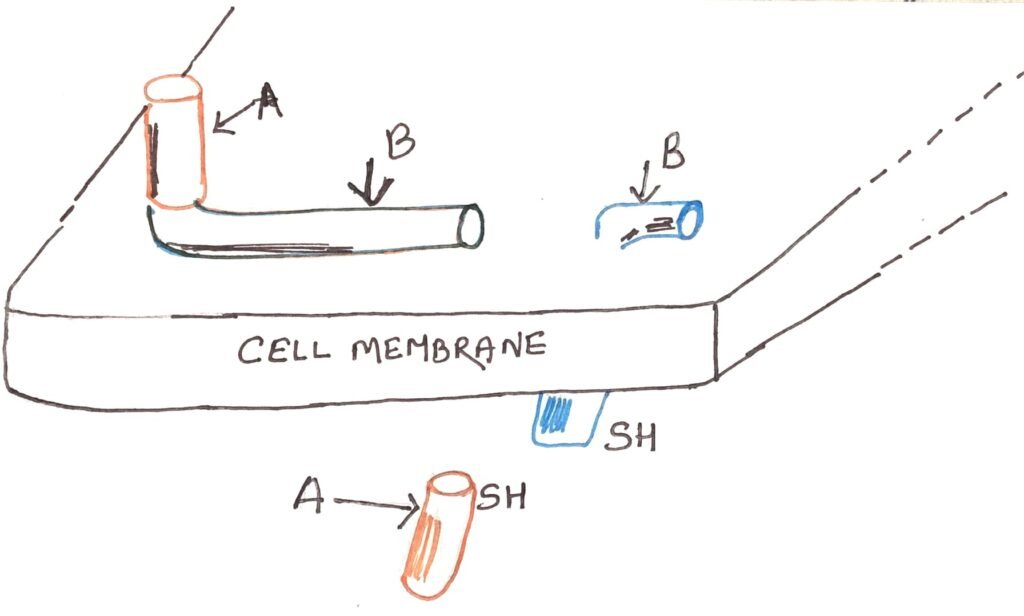
The diphtheria antitoxin, composed of antibodies that react with the toxin, can neutralize its activity by binding to the B subunit. Thus, it prevents the toxin from binding to host cells. However, once the toxin has already attached to a cell, the antitoxin becomes ineffective; only unbound toxin molecules can be neutralized.
Botulinum Toxin
Under normal conditions, voluntary muscle contraction occurs when a nerve impulse travels along the axon of a motor neuron and reaches the neuromuscular junction. It is a point where the nerve ending meets the muscle fiber. At this junction, the release of acetylcholine from the terminal ends of the axon triggers muscle contraction.
In botulism, however, the botulinum toxin binds to the axon terminals near the neuromuscular junction. The binding blocks the release of acetylcholine. As a result, muscle contraction is inhibited, leading to flaccid paralysis. When this paralysis spreads to the respiratory muscles, particularly the chest and diaphragm, it can cause death due to respiratory failure.
Cholera Toxin
The cholera toxin is composed of one A subunit and five B subunits. The B subunits enable the toxin to attach to the epithelial cells lining the small intestine. After which, the A subunit penetrates the cell membrane and is cleaved to form a smaller A₁ fragment. This fragment alters a regulatory protein that controls the enzyme adenylate cyclase, locking it in a permanently activated state. As a result, adenylate cyclase continuously converts ATP into cyclic AMP (cAMP):
ATP → 3′,5′-cAMP + pyrophosphate
The excessive production of cAMP causes a massive loss of water and electrolytes from intestinal cells into the intestinal lumen, producing severe diarrhea. This fluid loss also depletes bicarbonate ions in the blood, leading to acidosis. As dehydration worsens, blood proteins and red cells become concentrated, resulting in hypovolemic shock and possible circulatory collapse.
The primary treatment for cholera involves rapid replacement of fluids and electrolytes, typically through intravenous rehydration therapy. It compensates for the losses and prevents fatal dehydration.
Tetanus Toxin
Normal muscular movement depends on the coordinated action of two opposing muscles. For instance, when a person raises an arm, the biceps contract while the triceps relax and stretch. As the triceps stretch, stretch receptors within the muscle send signals to the central nervous system (CNS). This activates the motor neurons that control it. To prevent both muscles from contracting simultaneously, the CNS releases an inhibitory neurotransmitter, the amino acid glycine, which temporarily suppresses these motor neurons.
In tetanus, however, the tetanus toxin interferes with this mechanism by blocking the release of glycine from inhibitory neurons in the CNS. Without this inhibition, opposing muscle groups contract at the same time, producing the characteristic rigid spasms of tetanus. Sometimes it is so intense that they can fracture bones or tear tissues. Ultimately, death occurs due to respiratory failure, as the toxin prevents the normal function of the chest and diaphragm muscles required for breathing.
Mode of Action of Streptolysin O (SLO) and Streptolysin S (SLS)
Streptolysin O (SLO) is a toxin produced by Streptococcus pyogenes that is inactivated by oxygen. It exhibits multiple biological effects, primarily through its ability to damage cell membranes. SLO functions as a hemolysin, producing β-hemolysis around bacterial colonies on anaerobically incubated blood agar. However, during infection, its leucocidal activity( the ability to kill white blood cells) is more significant. The toxin does not require ingestion by leukocytes. Instead, it diffuses into the cells, disrupting the membranes of cytoplasmic granules and releasing lysosomal enzymes, which subsequently destroy the phagocyte. When administered intravenously to animals such as rabbits, SLO also acts as a cardiotoxin, rapidly disrupting the cardiac pumping cycle.
Streptolysin S (SLS), another toxin produced by S. pyogenes, exerts a similar leucocidal effect but differs in its properties. Unlike SLO, SLS is oxygen-stable and cell-bound, meaning it remains attached to the bacterial surface. As a result, it kills leukocytes only after the bacteria have been engulfed. Because of its oxygen stability, SLS can produce β-hemolysis even under aerobic conditions, distinguishing it from SLO.
Conclusion
Bacterial exotoxins represent some of the most potent biological substances known, each acting through distinct mechanisms to disrupt normal cellular and physiological processes. The diphtheria toxin inhibits protein synthesis by inactivating elongation factor-2, while botulinum toxin blocks the release of acetylcholine at neuromuscular junctions, causing flaccid paralysis.
In contrast, tetanus toxin prevents the release of inhibitory neurotransmitters such as glycine, leading to uncontrolled muscle contractions. The cholera toxin induces massive fluid and electrolyte loss from intestinal cells through the overactivation of adenylate cyclase, resulting in severe diarrhea and dehydration. Likewise, streptolysin O and streptolysin S, produced by Streptococcus pyogenes, damage cell membranes, destroy leukocytes, and cause hemolysis. Collectively, these toxins demonstrate the diverse strategies bacteria employ to establish infection and cause disease, highlighting the importance of understanding their mechanisms for the development of effective vaccines and therapeutic measures.
You may also like:
- Subunit vaccines– vaccines from purified macromolecules
- Microorganisms Develop Drug Resistance
- Endotoxins and Associated Virulence Determinants in Bacterial Pathogenesis

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.



