In this article, I briefly describe archaebacteria and their unique adaptations. Archaebacteria are ancient microorganisms that thrive in extreme environments such as high heat, salinity, and acidity. They include methanogens, halophiles, and thermoacidophiles, each adapted with unique metabolic and structural features for survival. Their remarkable enzymes and resilience make them vital to understanding early life and valuable for biotechnological innovations.
Archaebacteria: The Ancient Microbes with Diverse Forms and Habitats
Archaebacteria and eubacteria diverged very early in the evolutionary history of life on Earth. The significant phylogenetic gap between them is reflected in distinct differences in their physical and biochemical traits. Neither archaebacteria nor eubacteria form a uniform group, as both display considerable diversity in morphology, chemical composition, metabolism, and habitat.
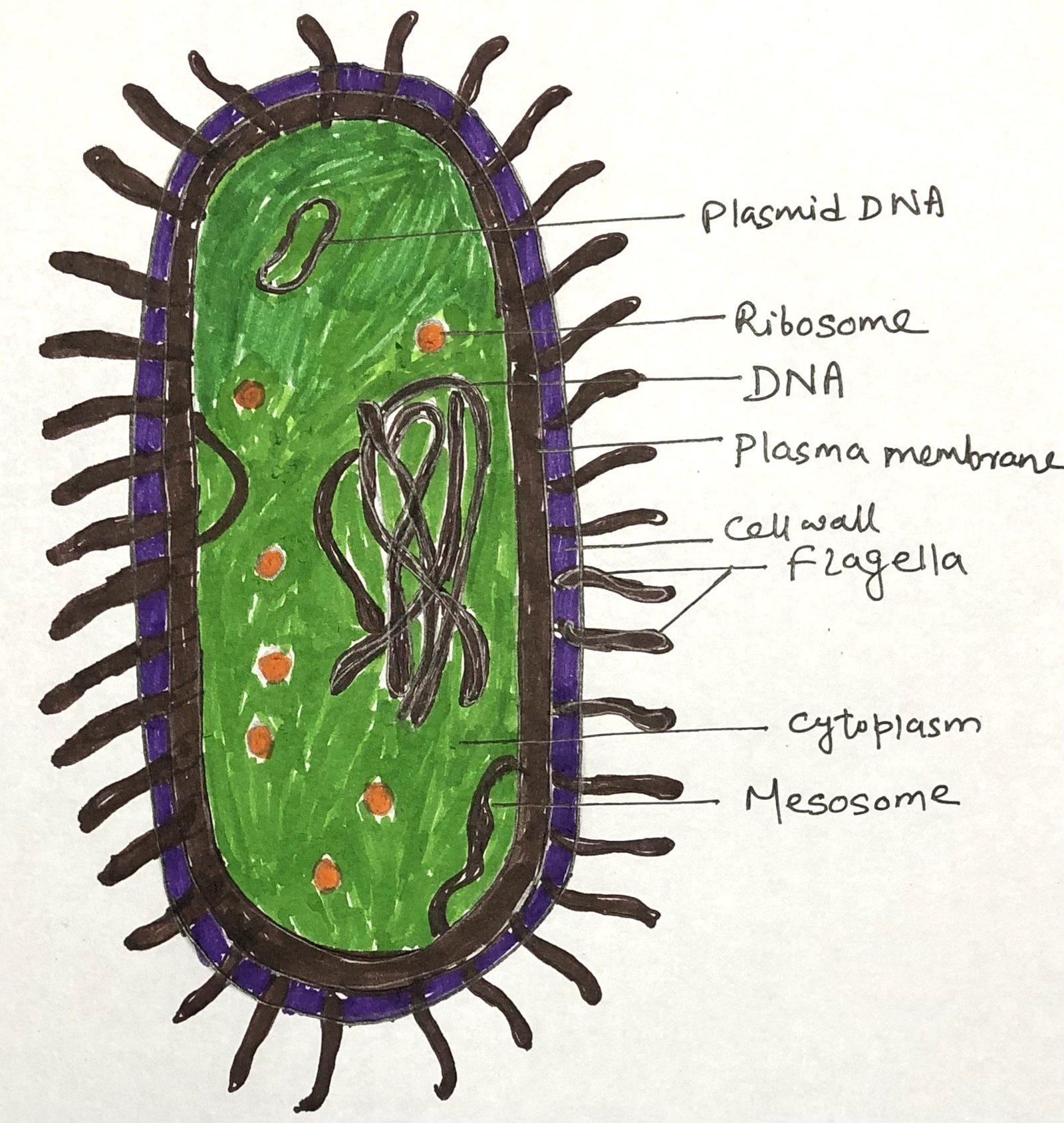
Archaebacteria are simple prokaryotes without a nucleus or membrane-bound organelles. Their cell wall lacks peptidoglycan and may contain pseudomurein or proteins, while the cell membrane has ether-linked lipids that resist extreme conditions. Many possess flagella-like structures (Figure 1) called archaella for movement, which differ in composition from bacterial flagella. They occur in various shapes, such as cocci, rods, or irregular forms, suited to their extreme habitats.
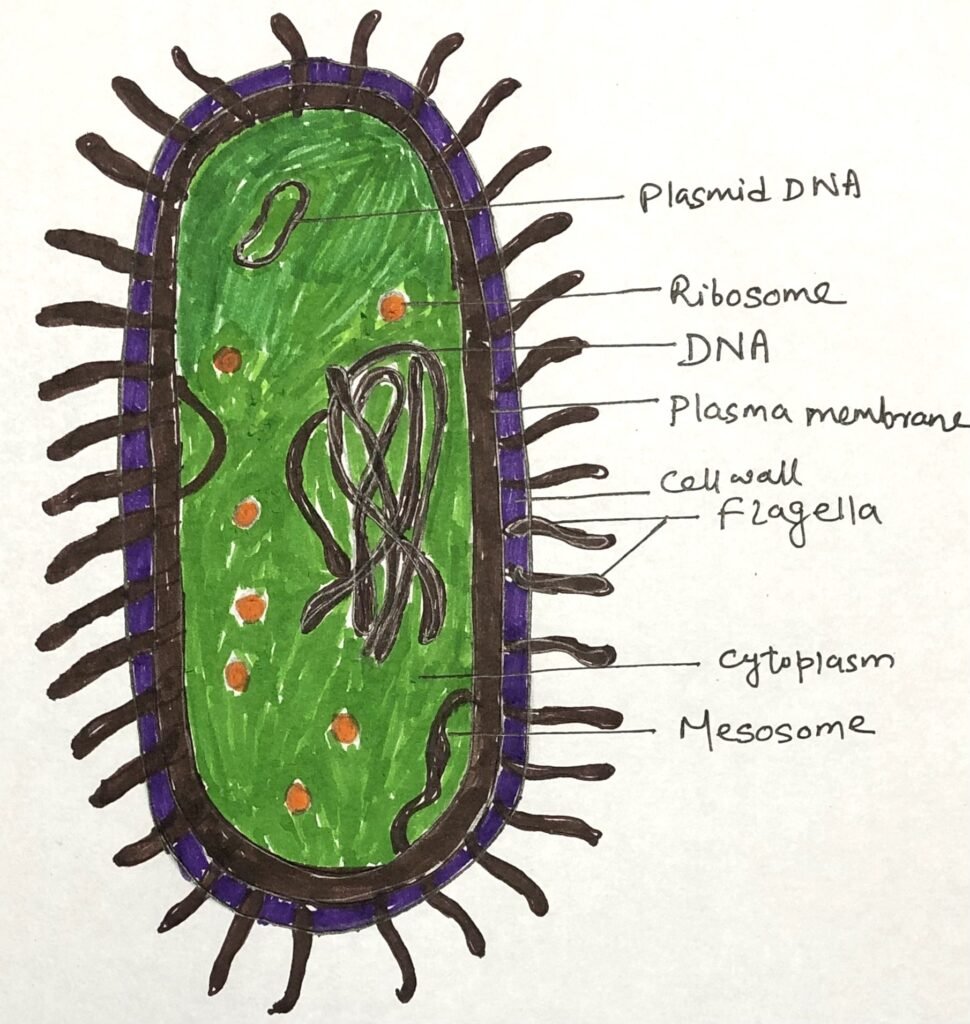
Among archaebacteria, three main types are recognized: methanogens (methane producers), extreme halophiles (salt-loving microbes), and thermoacidophiles(organisms that thrive in hot and acidic environments).
Metabolism and Unique Coenzymes of Methanogenic Archaebacteria
These archaebacteria are strict anaerobes capable of generating energy by oxidizing compounds such as hydrogen (H₂) or formate and using the electrons released in this process to reduce carbon dioxide (CO₂), resulting in the production of methane. Certain genera can grow autotrophically, relying solely on H₂ and CO₂ for both carbon and energy, while others require additional nutrients like vitamins, acetate, amino acids, or organic sulfur compounds. Generally, most species exhibit better growth in complex media, such as those containing yeast extract, compared to purely inorganic media.
Almost all methanogens possess two distinctive coenzymes not found in other bacteria. Coenzyme M participates in methyl transfer reactions, whereas coenzyme F420, a flavin-like compound, plays a role in their anaerobic electron transport system. Interestingly, coenzyme F420 fluoresces under ultraviolet light, allowing methanogens to be easily identified using a fluorescence microscope.
Classification and Habitats of Methanogenic Archaea
The genera of methane-producing bacteria, or methanogens, are classified based on their morphology and Gram staining characteristics. Variations in cell wall composition also serve as distinguishing features among these genera (Figure 2). In two of them, the cell wall is made of pseudomurein, a substance that differs from the typical eubacterial peptidoglycan. This difference arises from the replacement of N-acetylmuramic acid with N-acetyltalosaminuronic acid, and from the presence of a tetrapeptide chain composed entirely of L-amino acids, with glutamic acid at the terminal position.
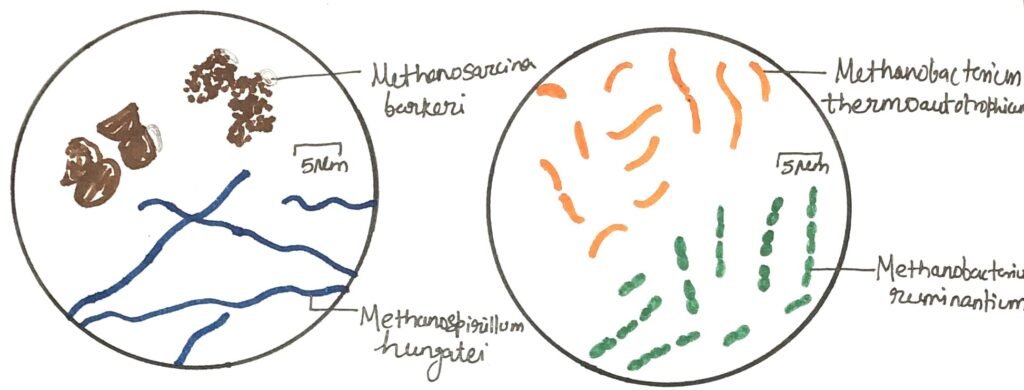
Methanogens thrive in anaerobic environments rich in organic matter, where non-methanogenic bacteria decompose material to release hydrogen (H₂) and carbon dioxide (CO₂)—the key substrates for methane production. Common habitats include marshes, swamps, pond and lake sediments, marine mud, the digestive tracts of humans and animals, the rumen of cattle, and anaerobic digesters used in sewage treatment plants.
Halophilic Archaea: Adaptations and Structural Features
These chemoorganotrophic, aerobic bacteria thrive in highly saline environments, requiring around 17–23% sodium chloride (NaCl) for optimal growth. They are Gram-negative and display various shapes, including rods, disks, and cocci. These microorganisms inhabit salt lakes, solar salt production sites, and salted protein-rich materials such as preserved fish. Their colonies exhibit red to orange pigmentation due to carotenoid compounds, which protect the cells from the harmful effects of sunlight.
To survive extreme salinity, the cells maintain a high internal concentration of potassium chloride (KCl), preventing dehydration. Their cytoplasmic membranes and ribosomes remain stable only in environments rich in KCl, and their enzymes are active exclusively at high levels of KCl or NaCl. The cell wall composition varies among genera. In Halobacterium, the wall is composed of protein subunits that remain intact only in the presence of sufficient salt; when the NaCl concentration drops below 10%, the cells lyse. In contrast, Halococcus possesses a complex heteropolysaccharide cell wall that remains stable even at low salt concentrations.
Energy Generation in Halobacteria
Halobacteria are primarily aerobic microorganisms, but they exhibit remarkable metabolic flexibility. In the absence of oxygen, they can produce ATP through the fermentation of the amino acid arginine, enabling limited anaerobic growth. Interestingly, these organisms also utilize a unique, light-dependent mechanism for energy production.
Under low-oxygen conditions, the cell membrane develops purple patches containing a pigment known as bacteriorhodopsin. When exposed to light, this pigment undergoes bleaching, during which protons are pumped outward across the membrane. The resulting proton motive force drives the synthesis of ATP, a process similar to that in phototrophic bacteria. However, unlike typical photosynthetic organisms, halobacteria lack bacteriochlorophyll—making their light-driven ATP generation a distinctive adaptation to extreme environments.
Thermoacidophiles- The Gram-negative Archaebacteria
Thermoacidophiles are a group of aerobic, Gram-negative archaea distinguished by their extraordinary ability to thrive in extremely hot and acidic environments. These microorganisms can survive at temperatures ranging from 60°C to over 90°C and in pH levels as low as 1–2, conditions that are lethal to most other life forms.
This group primarily includes two well-known genera — Thermoplasma and Sulfolobus. Thermoacidophiles are commonly found in volcanic hot springs, acidic sulfur pools, and geothermal vents, where both high temperature and acidity coexist. Their unique enzymes and stable biomolecules have attracted scientific interest for industrial and biotechnological applications, such as in high-temperature biocatalysis and acid-stable enzyme production.
Thermoplasma
Thermoplasmas are chemoorganotrophic archaea that share a strong resemblance to mycoplasmas, as both groups lack a rigid cell wall and produce small, “fried-egg”–shaped colonies on culture media. Their cells are highly pleomorphic, exhibiting a wide range of shapes, from spherical to filamentous, depending on growth conditions.
These organisms thrive at an optimum temperature of 55–59°C and an acidic pH of around 2, conditions under which few other microorganisms can survive. When exposed to neutral or alkaline environments, their cells rapidly undergo lysis due to the absence of a stabilizing cell wall.
Thermoplasmas have been isolated from burning coal refuse heaps and other self-heating, sulfur-rich environments where both high temperature and acidity coexist. Their flexible, lipid-rich plasma membrane, reinforced by tetraether lipids, provides structural stability under such extreme conditions. Because of their resilience, Thermoplasmas serve as models for studying cell membrane adaptations and are of growing interest in industrial biotechnology for the development of heat- and acid-tolerant enzymes.
Sulfolobus
Members of the genus Sulfolobus are spherical or irregularly lobe-shaped archaea that thrive in extremely hot and acidic environments. Unlike Thermoplasma, their cells possess a distinct protein-based cell wall, which provides rigidity and protection under harsh conditions. Most species grow optimally at temperatures between 70°C and 87°C and at an acidic pH of around 2, conditions typical of volcanic hot springs and acidic geothermal vents, where they are often the dominant microbial inhabitants.
Sulfolobus species exhibit metabolic versatility. They are facultatively autotrophic, meaning they can utilize different nutritional modes depending on environmental availability. Under certain conditions, they act as chemolithotrophs, oxidizing elemental sulfur or sulfide compounds to derive energy and producing sulfuric acid as a by-product. Alternatively, they can grow chemoorganotrophically by metabolizing organic substrates such as sugars or amino acids.
Because of their thermal and acid stability, Sulfolobus species have become important model organisms in research on extremophile biology, DNA repair mechanisms, and enzyme adaptation. Their enzymes, particularly DNA polymerases and sulfur-oxidizing enzymes, are valuable in biotechnology and industrial processes that require high-temperature, acid-resistant catalysts.
Conclusion
Archaebacteria represent one of the most ancient and resilient forms of life on Earth, adapted to survive in extreme environmental conditions that would be lethal to most other organisms. Their remarkable diversity reflects distinct physiological and biochemical adaptations. Methanogens thrive in anaerobic habitats such as marshes and animal intestines, producing methane through the reduction of carbon dioxide. Halophiles, including Halobacterium and Halococcus, flourish in highly saline environments where they maintain osmotic balance using potassium ions and, in some cases, generate ATP through a unique light-driven mechanism involving bacteriorhodopsin. On the other hand, thermoacidophiles, such as Thermoplasma and Sulfolobus, inhabit extremely hot and acidic niches like volcanic springs and burning coal heaps. While Thermoplasma lacks a cell wall and depends on a lipid membrane for stability, Sulfolobus possesses a proteinaceous wall and oxidizes sulfur compounds for energy.
Together, these groups illustrate the extraordinary adaptability of Archaebacteria, showcasing a wide range of metabolic strategies—from methanogenesis to photophosphorylation and sulfur oxidation. Their unique enzymes and biomolecules not only reveal insights into the early evolution of life but also hold immense potential for biotechnological and industrial applications, particularly in processes requiring extreme heat, salinity, or acidity tolerance.
You may also like:

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.