In this article, I briefly describe how antibiotics inhibit nucleic acid and protein synthesis.
Antibiotics
The term “antibiotic” describes a metabolic compound produced by microorganisms that can inhibit or destroy other microbes, even in minimal amounts. Vuillemin first introduced the idea of antibiosis in 1898, referring to a phenomenon where one organism eliminates others to ensure its own survival. However, this initial concept differs from the modern definition put forward by Waksman in 1945. Waksman defined antibiotics as chemical substances derived from microorganisms that exhibit antimicrobial activity at low concentrations.
Antibiotics and Their Modes of Action
Antibiotics can be grouped based on their chemical composition and how they function. In terms of function, they are divided into bactericidal agents, which kill bacteria, and bacteriostatic agents, which prevent bacterial growth. Classification by mode of action focuses on the specific processes these drugs target within microbial cells. They work by disrupting key cellular activities such as building the cell wall, damaging the cell membrane, blocking the synthesis of DNA, RNA, or proteins, or interfering with enzymes crucial for the microorganism’s survival.
Some antibiotics disrupt the production of nucleic acids and proteins, processes that involve complex biochemical pathways. Drugs such as streptomycin, tetracyclines, chloramphenicol, and erythromycin are known to interfere with these essential cellular functions.
Streptomycin
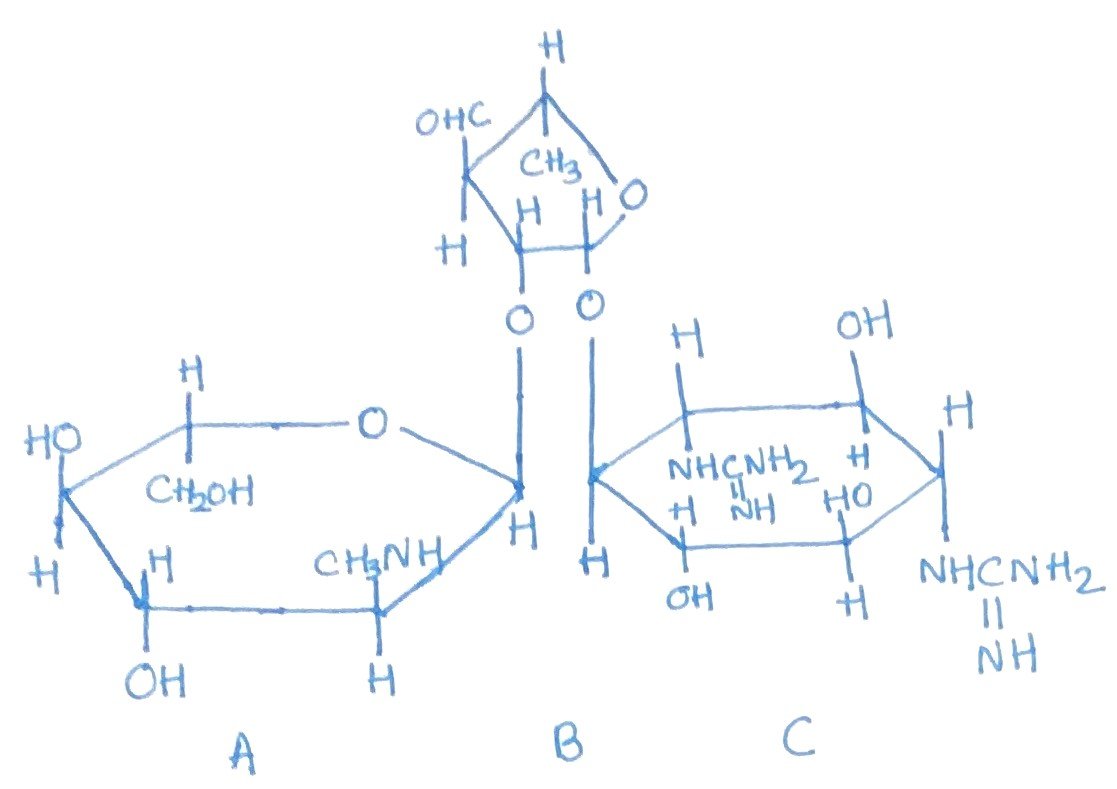
Streptomyces griseus, a soil-dwelling microorganism, is the natural source of the antibiotic streptomycin. This antibiotic was first isolated in 1944 by Schatz, Bugie, and Waksman. Streptomyces griseus is effective against a group of bacteria resistant to sulfonamides and penicillin. Its antibacterial range includes various Gram-negative species, such as Francisella tularensis and certain members of the Salmonella genus. When highly purified, streptomycin is safe for humans and animals in small doses. However, prolonged use can lead to cumulative damage in a specific area of the nervous system.
Streptomycin belongs to the class of aminoglycoside antibiotics (Figure 1). Other examples in this group include kanamycin, which is produced by Streptomyces kanamyceticus, and neomycin, produced by Streptomyces fradiae and other Streptomyces species. These antibiotics, including streptomycin, interfere with protein synthesis by binding irreversibly to the 30S subunit of mRNA, thereby disrupting the normal process of protein formation.
Tetracyclines
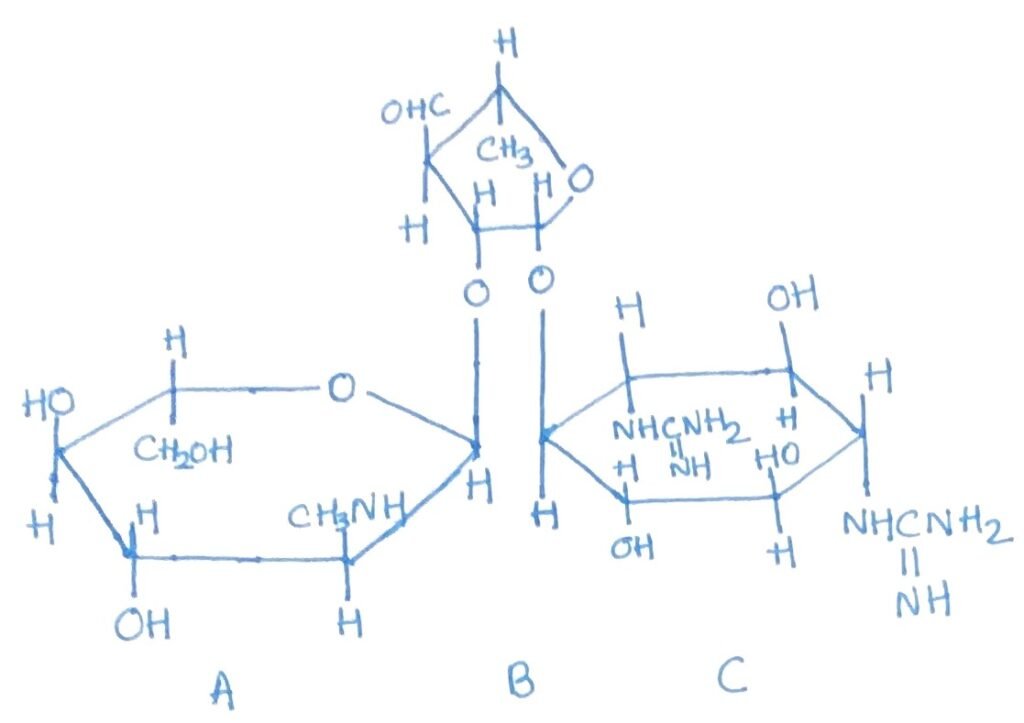
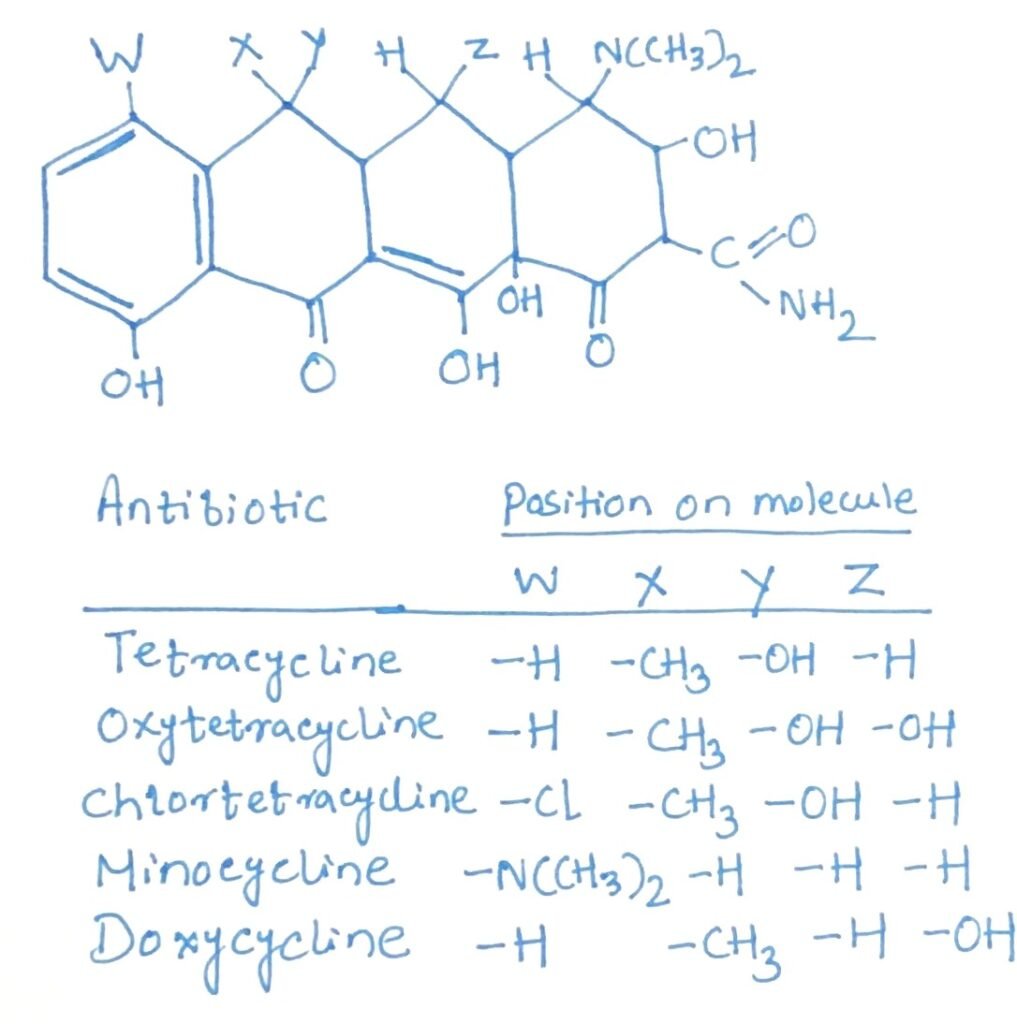
These are broad-spectrum antibiotics with comparable antimicrobial activity, and bacterial cross-resistance among them is common. The five main types—chlorotetracycline, oxytetracycline, tetracycline, doxycycline, and minocycline—share similar chemical and biological characteristics. Chlorotetracycline is produced by Streptomyces aureofaciens, while Streptomyces rimosus yields oxytetracycline. As dry powders, the hydrochloride and base forms of tetracyclines are highly stable, though their stability varies in solution; tetracycline remains active for over three weeks, whereas chlorotetracycline and oxytetracycline are less stable.
Tetracycline, oxytetracycline, doxycycline, and minocycline are chemically alike (Figure 2), exhibit similar antimicrobial spectra, and function as bacteriostatic agents. Resistance to one typically indicates resistance to the others. Tetracycline is known for its low toxicity in laboratory animals and is effectively absorbed from the gastrointestinal tract, making it suitable for oral administration. These antibiotics act by inhibiting protein synthesis, specifically by preventing aminoacyl-tRNA from binding to the 30S ribosomal subunit.
Chloramphenicol
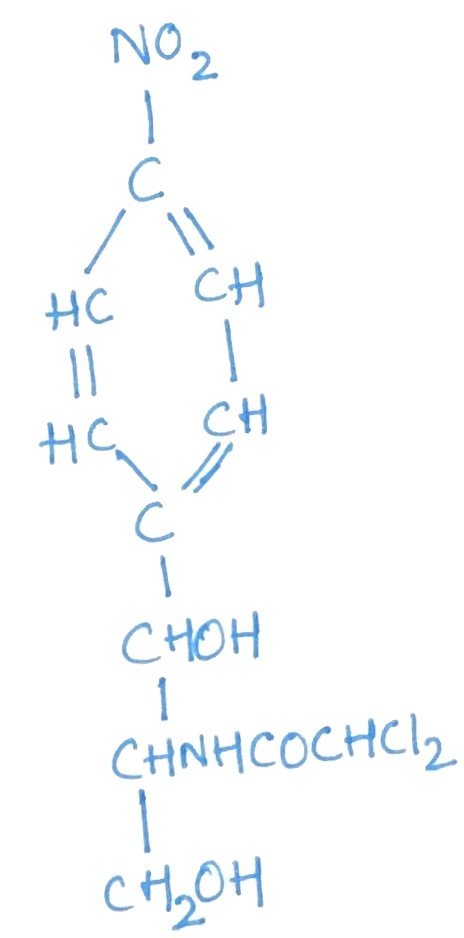
Chloramphenicol is a broad-spectrum antibiotic effective against a wide range of both Gram-positive and Gram-negative bacteria, sharing a similar antimicrobial spectrum with tetracycline. Like tetracycline, it acts as a bacteriostatic agent. Structurally, it features a nitrobenzene ring and a non-ionic chlorine group (Figure 3). However, its use as a general antibacterial agent is limited due to the risk of serious side effects, such as blood dyscrasias. Chloramphenicol exerts its effect by binding to the 50S ribosomal subunit, where it inhibits protein synthesis by blocking transpeptidation and translocation processes.
Erythromycin
Erythromycin is an antibiotic produced by the bacterium Streptomyces erythraeus. It is effective against Gram-positive bacteria, certain Gram-negative strains, and pathogenic spirochetes. Its antimicrobial spectrum and clinical applications are similar to those of penicillin. However, erythromycin has the added advantage of being effective against bacteria that have developed resistance to penicillin and streptomycin. As a result, it is commonly prescribed for patients who are allergic to penicillin.
Belonging to the macrolide class of antibiotics, erythromycin features a large lactone ring structure that is connected to amino sugars via glycosidic bonds. Its action involves inhibiting protein synthesis by binding to the 50S subunit of the bacterial ribosome, thereby blocking the processes of transpeptidation and translocation during protein assembly.
Conclusion
Antibiotics are microbial compounds that inhibit or kill other microbes at low concentrations. The concept began with Vuillemin’s 1898 idea of antibiosis and was later defined more precisely by Waksman in 1945. Antibiotics target vital cellular processes like cell wall formation, membrane integrity, and the synthesis of DNA, RNA, proteins, or key enzymes. Drugs like streptomycin, tetracyclines, chloramphenicol, and erythromycin disrupt nucleic acid and protein production.
Streptomyces griseus is effective against a range of bacteria resistant to sulfonamides and penicillin. Its antibacterial range includes various Gram-negative species, such as Francisella tularensis and certain members of the Salmonella genus.
Tetracyclines are broad-spectrum antibiotics with comparable antimicrobial activity, and bacterial cross-resistance among them is common. The five main types—chlorotetracycline, oxytetracycline, tetracycline, doxycycline, and minocycline—share similar chemical and biological characteristics. Chloramphenicol is a broad-spectrum antibiotic effective against a wide range of both Gram-positive and Gram-negative bacteria, sharing a similar antimicrobial spectrum with tetracycline. Erythromycin is an antibiotic produced by the bacterium Streptomyces erythraeus. It is effective against Gram-positive bacteria, certain Gram-negative strains, and pathogenic spirochetes.
You may also like:

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.