In this article, I briefly explain the disparity in immunity in the small and large intestines.
Distinct Immunity in Small and Large Intestine
The small and large intestines manage their relationship with the microbiome by sharing general strategies. However, the physiology of each section is distinct, as are the microbial communities and immune cells that inhabit them.
The small intestine hosts a smaller and less varied community of commensal microbes than the large intestine. In contrast, the large intestine harbors the densest and most diverse microbial population. The small intestine’s lumen contains fewer than a million commensal bacteria per milliliter of fluid. The large intestine’s lumen can hold between one billion and one trillion bacteria per milliliter.
Small and Large Intestines React to Different Pathogens
Pathogens differ in their stay in the small and large intestines. A bacterium, Clostridium difficile, linked with diarrhea outbreaks in hospitals, is found in the large intestine. Norovirus, linked with diarrhea outbreaks on cruise ships, attacks the small intestine. Whipworm infects the large intestine, whereas the roundworm infects the small intestine.
Small and large intestines vary in their susceptibility to various inflammatory disorders and cancers. Inflammatory reactions in the small intestine lead to the cause of celiac disease, and inflammation in the large intestine causes ulcerative colitis. The large intestine involves more tumors compared to the small intestine.
Epithelial cells also vary in concentrations in the small and large intestines. The small intestine has many Paneth cells, whereas the large intestine has few of them. The large intestine has many goblet cells, which help generate a much thicker and a protective mucus layer. The small intestine exclusively includes Peyer’s patches and their associated M cells. Isolated lymphoid follicles are more prevalent in the large intestine, which lacks villi and, unlike the small intestine, is not primarily involved in food digestion and nutrient absorption.
Commensal Microbes Contribute to Sustaining a Tolerogenic Environment in the Intestine
Microbes that co-evolved with humans shaped the development of our immune receptors, including invariant T-cell receptors with fixed specificities for common microbial antigens. Microbes that colonize our gut early in life help to regulate and fine-tune our immune system by promoting the development of regulatory T cells and encouraging the production of IgA antibodies that target commensal bacteria.
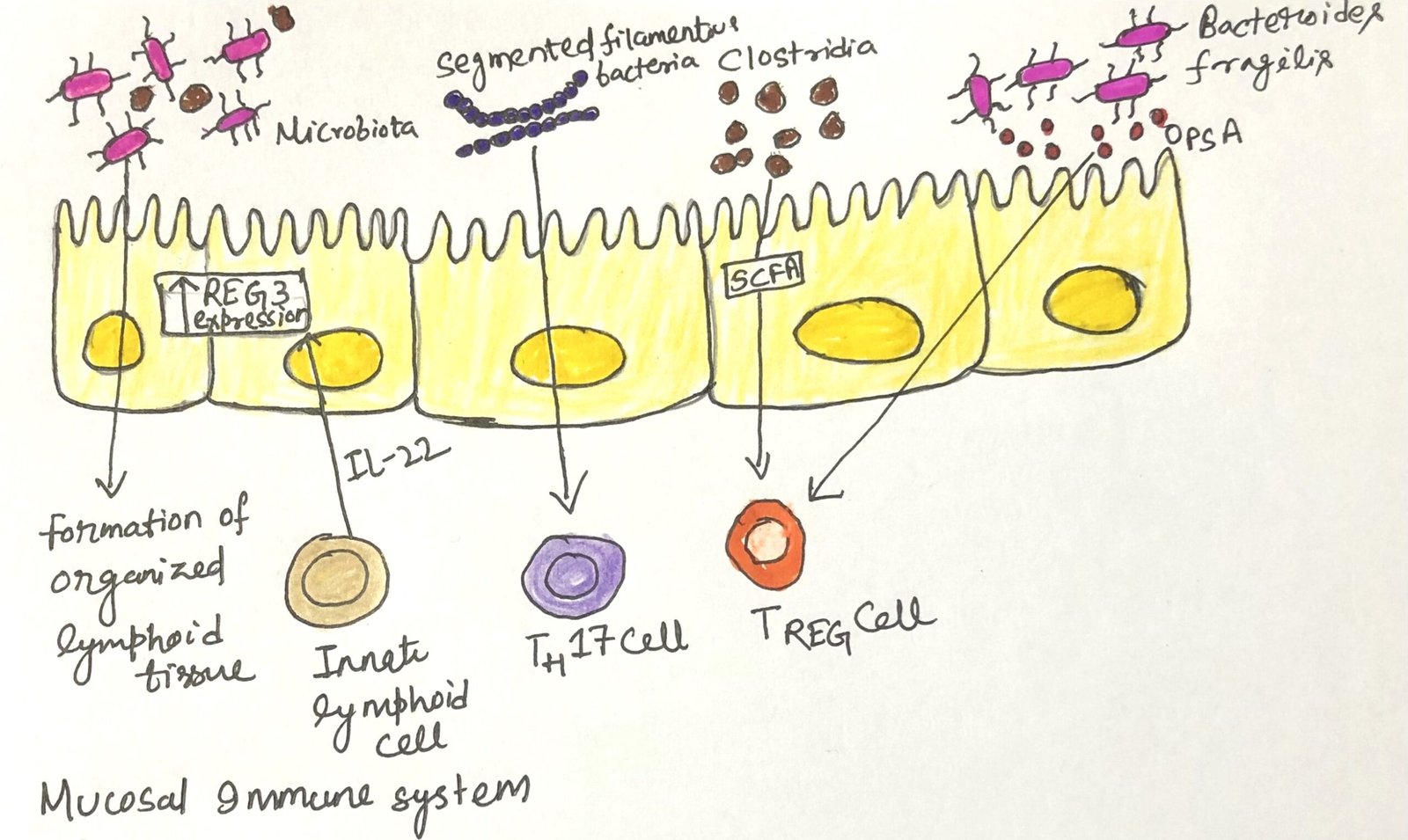
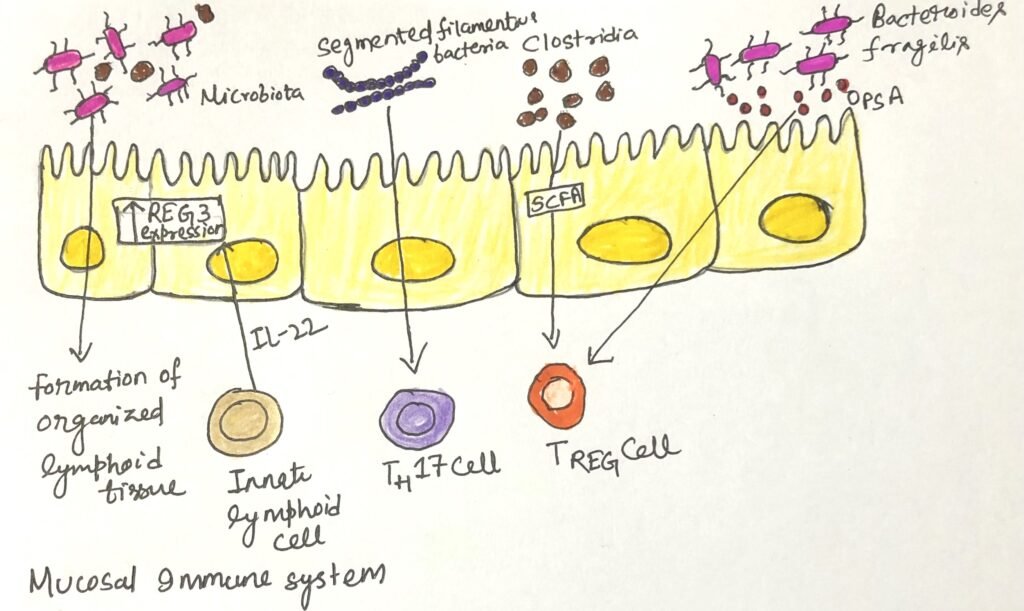
Researchers have discovered that certain species of commensal bacteria are especially effective at promoting the accumulation of regulatory T cells and enhancing immune tolerance (Figure 1). The bacterial species include the phyla Firmicutes, Actinobacteria, and Bacteroidetes, all are included in a healthy human microbiome. Bacteria in these phyla ferment dietary fiber and produce short-chain fatty acids (SCFAs). The short-chain fatty acids directly influence dendritic cells in the gut and enhance the development of regulatory T cells. Another member of the Firmicutes phylum colonizes the small intestine and enhances the production of IgA and the development of TH17.
Viruses Positively Impact the Development of the Intestine
Recent studies indicate that exposure to viruses also positively impacts intestinal development and improves systemic immune function. In an experiment, when germ-free mice, which have a more permeable intestinal epithelial barrier and a mucosa lacking immune cells, are exposed to just one viral species, their immune cell populations are replenished, the connections between epithelial cells are reinforced, and immune balance in the gut is restored. This process is partly driven by the virus interacting with pattern recognition receptors, which initiates a cascade of events that support healthy, tolerogenic immune activity in the intestine.
Commensal Gut Microbiome Impacts the Immune System
Our commensal gut microbiome, along with tolerizing our gut immune system, influences the entire immune system too. When tolerogenic responses in the gut are compromised, autoimmune reactions are more likely to occur in other parts of the body. For example, individuals with systemic lupus erythematosus tend to have a microbiome that encourages inflammatory responses and may benefit from supplementation with Bifidobacterium, a bacterium from the phylum Actinobacteria. Introducing certain microbes through probiotic consumption or fecal transplants could improve intestinal health, along with boosting both the gut and systemic immune systems. Fecal transplantation, which involves introducing a healthy donor’s colonic microbiome into an infected individual, can surpass harmful bacteria and restore the integrity of the intestinal lining.
Conclusion
The small intestine hosts a smaller and less varied community of commensal microbes compared to the large intestine. Pathogens differ in their stay in the small and large intestines. Small and large intestines vary in their susceptibility to various inflammatory disorders and cancers. Inflammatory reactions in the small intestine are the cause of celiac disease. Inflammation in the large intestine causes ulcerative colitis. Epithelial cells also vary in concentrations in the small and large intestines. Isolated lymphoid follicles are more prevalent in the large intestine, which lacks villi. The large intestine, unlike the small intestine, is not primarily involved in food digestion and nutrient absorption.
Microbes that colonize our gut early in life help to regulate and fine-tune our immune system. They promote the development of regulatory T cells and encourage the production of IgA antibodies that target commensal bacteria. Certain species of commensal bacteria are especially effective at promoting the accumulation of regulatory T cells and enhancing immune tolerance. Our commensal gut microbiome, along with tolerizing our gut immune system, influences the entire immune system too.
You may also like:
- Various innate and adaptive cell types promote immune homeostasis in the intestine
- Common intestinal diseases
- The intestinal immune system recognizes and responds to pathogens
- Parallelism between the intestinal immune system and the respiratory immune system
- Normal Flora of the Intestinal Tract

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.