In this article, I briefly describe how various innate and adaptive cell types promote immune homeostasis in the intestine.
Our Gastrointestinal Tract
It is a tube-like structure running from the mouth to the anus. The gastrointestinal tract maintains our commensal microbiome and regulates local and systemic immune responses. Our gastrointestinal tract deals with billions of microorganisms, including viruses, bacteria, and parasitic worms. The small intestine receives partially digested food from the stomach and is the major site of most digestion and absorption. It is divided into three sections: duodenum, jejunum, and ileum. The undigested food from the small intestine enters the large intestine or colon. The large intestine absorbs water with some vitamins and delivers waste to the rectum and anus.
Immune Homeostasis in the Intestine
Several innate and adaptive cell types promote immune homeostasis. Our intestinal immune system has evolved an extensive system of sampling the microbiome and transferring microbes to antigen-presenting cells. Thus, they can be further evaluated by the adaptive immune system. Specialized epithelial cells like goblet cells and Paneth cells produce mucus and inhibit bacterial mobility. Epithelial cells also generate a wide range of antimicrobial peptides and other proteins, including defensins, which create pores in bacterial membranes. B cells in the intestinal mucosa secrete IgA, which is transcytosed from the lamina propria into the lumen, where it interacts with commensal microbes.
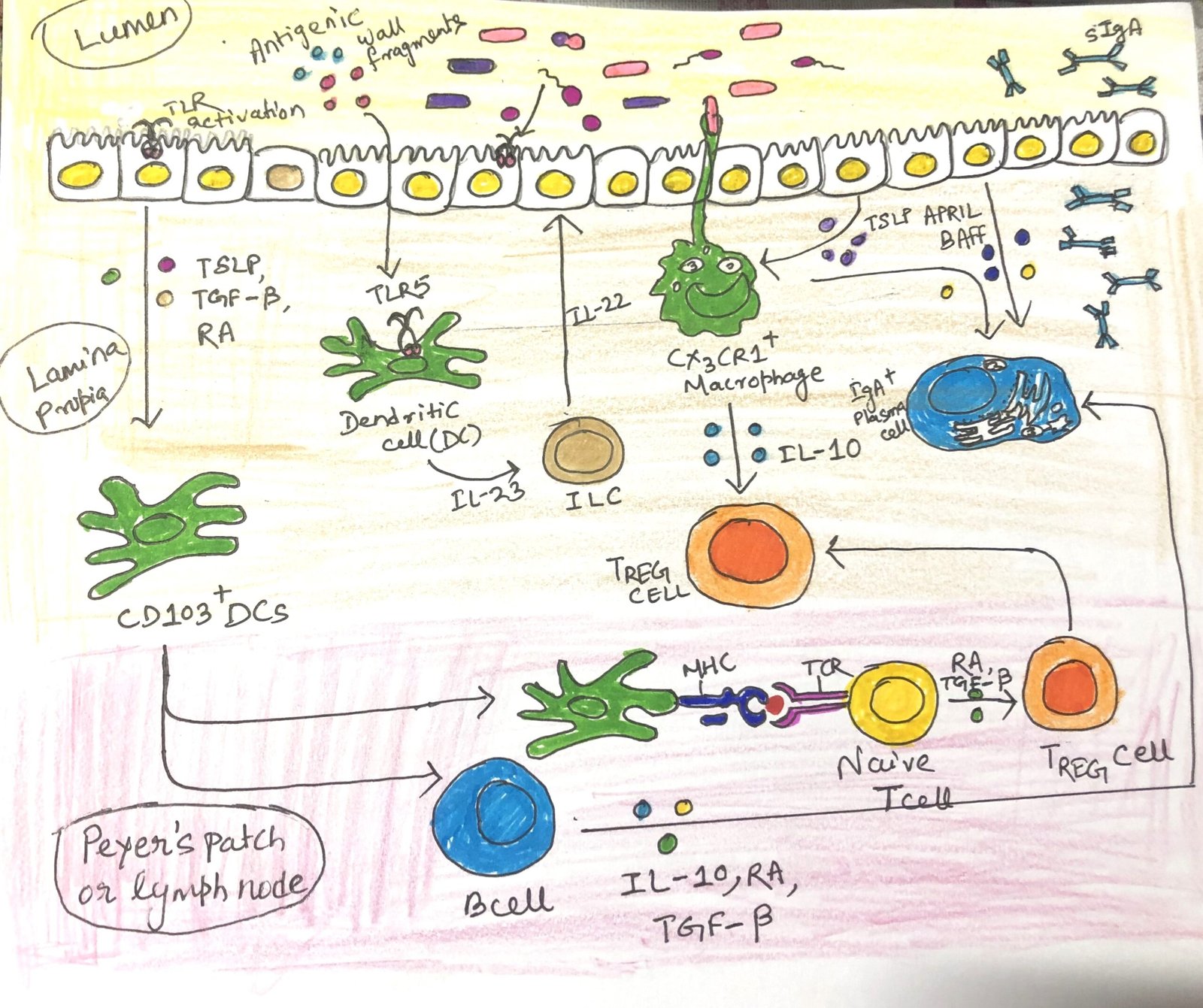
Under normal conditions, commensal microbes activate epithelial cells through pattern recognition receptors, such as Toll-like receptors (TLRs). This interaction leads to the release of TGF-β, retinoic acid (RA), a metabolite of vitamin A, and thymic stromal lymphopoietin. Together, these molecules help maintain an immune-tolerant environment by directing antigen-presenting cells to promote the differentiation of T cells into the regulatory T cell (TREG) lineage.
Some epithelial cells and antigen-presenting cells produce a B-cell activating factor and a proliferation-inducing ligand, APRIL, which promotes IgA production by lamina propria B cells (Figure 1). IL-10 is a potent anti-inflammatory cytokine and is ubiquitous in the healthy mucosa. It is known to be produced by T cells, macrophages, dendritic cells, and B cells. Individuals with lower levels of IL-10 are susceptible to inflammatory bowel disease. The cell subsets that play the most prominent role in maintaining gut homeostasis are TREG cells, IgA-secreting B cells, T follicular helper (TFH) cells, TH17 cells, ILC3s, and intraepithelial lymphocytes (IELs).
TREG Cells Generation
Activated dendritic cells meet circulating naïve T cells at the mesenteric lymph nodes or Peyer’s patches. When dendritic cells first meet their antigen in the tolerizing cytokine environment, they turn T-cell differentiation toward the TREG lineage. TREG cells (Fig 1) represent 30% of all T cells in the gut lamina propria. Individuals with low TREG populations are susceptible to a variety of immune-mediated diseases, including colitis.
The two types of regulatory T cells have two distinct sources. Thymic TREGS develop in the thymus, whereas peripheral TREGS are generated from naïve T cells activated by tolerogenic antigen-presenting cells. Peripheral TREGS activated by gut antigen-presenting cells are often induced to express gut-homing receptors. After expressing, they travel back to the intestinal mucosa, where they release IL-10 that reinforces tolerance.
Generation of B cells Secreting IgA
B cells producing IgA antibodies are found in abundance in intestinal tissues. IgA antibody is the first line of defense against pathogenic microbes and toxins. It also transports antigens from the lumen to the lamina propria for further evaluation.
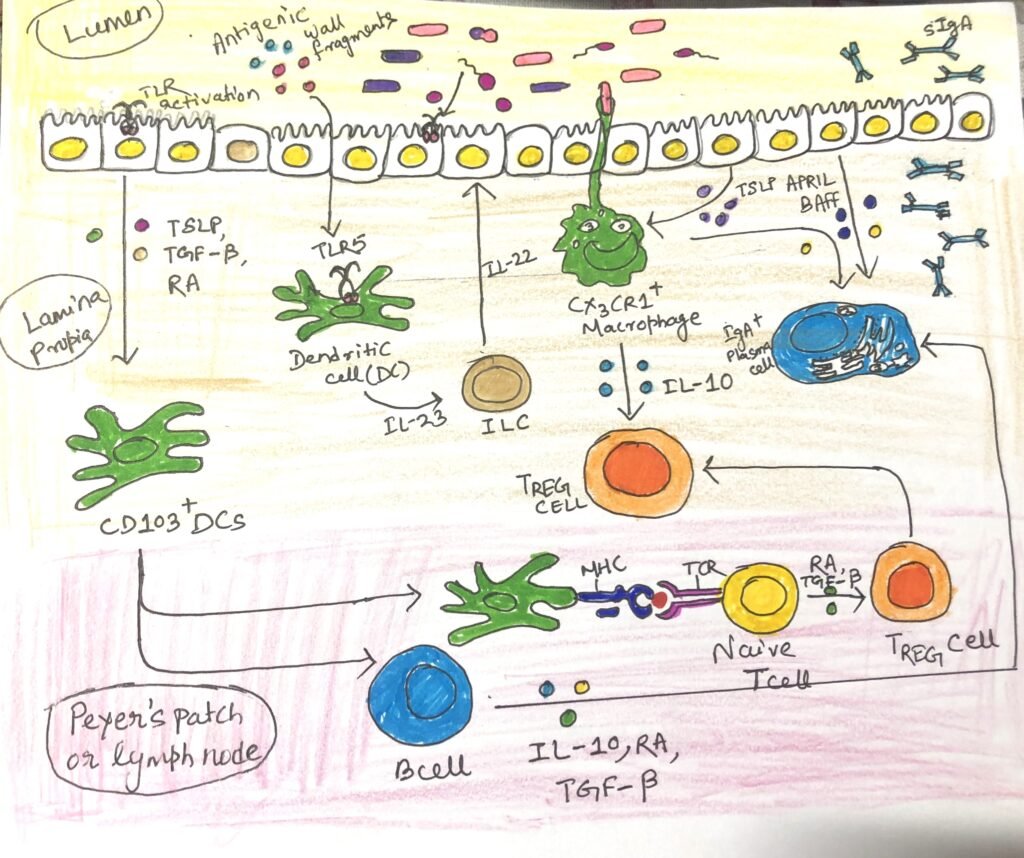
IgA antibodies come from different sources, such as some that come from B cells activated in B cell follicles in the Peyer’s patches of the small intestine. Many IgA antibodies come from B cells in isolated lymphoid follicles and B cells activated in draining mesenteric lymph nodes. IgA class switching happens through T-dependent and T-independent mechanisms and is influenced by the specific cytokine environment produced by immune cells in the intestine (Figure2 and Figure3).
T Cell-Dependent Class Switching and T Cell-Independent IgA Induction
T cell-dependent class switching to IgA begins with the activation of B cells by antigen follicles of mesenteric lymph nodes or Peyer’s patches. They undergo somatic hypermutation and class switching in the germinal center generated by them. The follicular helper T cells (TFH) provide a combination of signals to the B cells that favor IgA switching, including CD40L and the key cytokine TGF-β (Figure 2). Surprisingly, intestinal TH17 and TREG cells can convert to TFH cells that induce IgA class switching. B cells that mature successfully in lymphoid tissue differentiate into plasma cells that express the adhesion molecule α4β7 and the chemokine receptor CCR9 (Figure 2), enabling them to migrate to the mucosal sites. This process takes approximately one week to produce IgA antibodies.
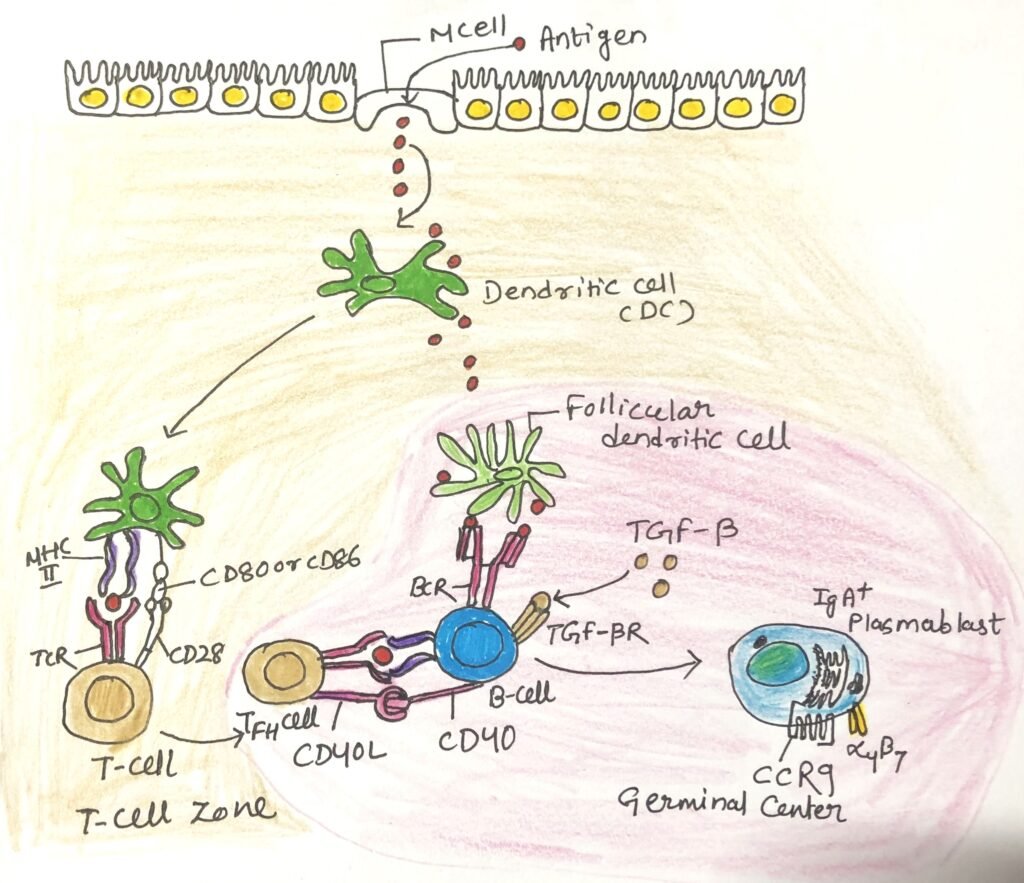
IgA antibodies can also be produced in a T-independent manner (Figure 3), which is quicker than the T-dependent manner. This process depends on the cytokines BAFF and APRIL, produced by stimulated epithelial cells and mucosal dendritic cells. In this manner, IgA antibodies with lower affinity are produced.
TH17 cells and ILC3 play an Important Role in Intestinal Immune Homeostasis
In the gut mucosa, the ILC3 subset plays an important role in maintaining tolerance. Innate immune cells produce a variety of signals, including IL-23 and RA. In response to these signals, ILC3 cells produce IL-17 and IL-22 (Figure 1). ILC3s are now recognized as the primary source of intestinal IL-22, which promotes the secretion of antimicrobial peptides, particularly REG3, by epithelial cells. Additionally, ILC3s boost B-cell production of IgA both directly, by releasing molecules that encourage B-cell differentiation, and indirectly by facilitating the formation of isolated lymphoid follicles. ILC1s and ILC2s play an important role in the gut response to pathogens.
TH17 cells, like ILC3 cells, are found in plenty in the healthy gut lamina propria. They maintain barrier immunity by secreting IL-22 and enhancing the health and activity of epithelial cells. The cytokines produced by ILC3s and TH cells have the potential to elevate inflammation and cause disease. The cytokine milieu may play a key role in balancing the health-promoting and inflammation-promoting functions of ILC3s and TH17. The tolerogenic cytokines IL-10 and TGF-β support the development of anti-inflammatory TH17 cells. However, when the pro-inflammatory cytokine IL-6, produced by various cell types during infection, is present, it can drive TH17 cells to adopt more inflammatory functions.
Intraepithelial Lymphocytes Play a Key Role in Intestinal Immune Homeostasis
TCR αβ and TCR γδ are the intraepithelial lymphocytes, which are crucial for intestinal immunity. They reside between epithelial cells, positioned beneath their tight junctions. While these lymphocytes can be mobilized in a typical immune response against invading pathogens, they also contribute to homeostasis. They help preserve the integrity of the epithelial barrier by secreting antimicrobial peptides and immunosuppressive cytokines.
The natural intraepithelial lymphocytes directly come from the thymus and reside in the intestine. Induced intraepithelial lymphocytes arise from naïve T cells activated within the gut-associated lymphoid tissue.
Conclusion
Several innate and adaptive cell types promote immune homeostasis. Our intestinal immune system has evolved an extensive system of sampling the microbiome and transferring microbes to antigen-presenting cells. Thus, they can be further evaluated by the adaptive immune system.
Under normal conditions, commensal microbes activate epithelial cells through pattern recognition receptors, such as Toll-like receptors (TLRs). The cell subsets that play the most prominent role in maintaining gut homeostasis are TREG cells, IgA-secreting B cells, T follicular helper (TFH) cells, TH17 cells, ILC3s, and intraepithelial lymphocytes (IELs).
TREG cells represent 30% of all T cells in the gut lamina propria. The two types of regulatory T cells have two distinct sources. Thymic TREGS develop in the thymus, whereas peripheral TREGS are generated from naïve T cells activated by tolerogenic antigen-presenting cells.
IgA antibodies come from different sources, such as some that come from B cells activated in B cell follicles in the Peyer’s patches of the small intestine. Many IgA antibodies come from B cells in isolated lymphoid follicles and B cells activated in draining mesenteric lymph nodes.
TH17 cells and ILC3 cells are found abundantly in the healthy gut lamina propria. They maintain barrier immunity by secreting IL-22 and elevating the health and activity of epithelial cells. The cytokines produced by ILC3s and TH cells have the potential to enhance inflammation and cause disease.
TCR αβ and TCR γδ are the intraepithelial lymphocytes that reside between epithelial cells, positioned beneath their tight junctions. While these lymphocytes can be mobilized in a typical immune response against invading pathogens, they also contribute to homeostasis.
You may also like:
- Small and large intestines possess different immune systems
- Type I Hypersensitivity and the Role of Genes and Environment in Allergy

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.