In this article, I provide a brief explanation of the β-adrenergic receptor-mediated signal transduction process, which involves adenylyl cyclase, cAMP, and PKA. β-adrenergic receptor-mediated signal transduction is a key cellular communication pathway activated by adrenaline or noradrenaline. Upon ligand binding, the receptor stimulates adenylyl cyclase via a G-protein, leading to increased cAMP production, which in turn activates protein kinase A (PKA) to regulate various physiological responses.
The β-adrenergic Receptor
The adrenergic receptors are categorized into four major types—α1, α2, β1, and β2—based on their differing affinities for agonists and antagonists, as well as the physiological responses they mediate. Agonists are molecules that bind to these receptors and mimic the action of the natural ligand, triggering a cellular response. In contrast, antagonists bind to the receptor without activating it, thereby blocking the effects of both the natural ligand and agonists.
The β-adrenergic receptors are crucial in tissues such as skeletal muscle, liver, and adipose tissue, where they regulate various aspects of fuel metabolism in response to the hormone epinephrine.
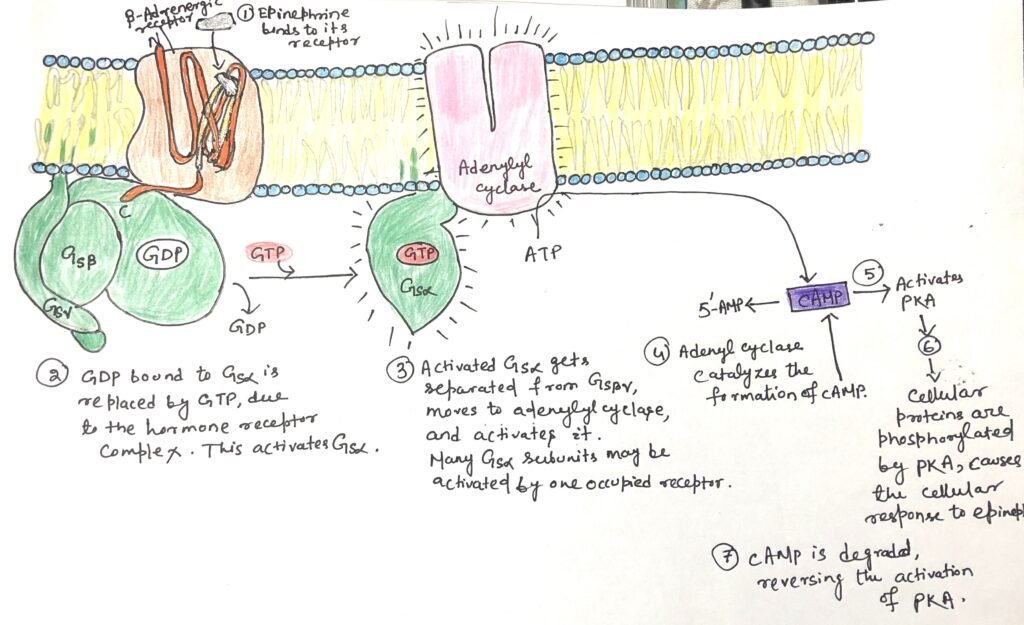
These receptors belong to the family of G-protein-coupled receptors (GPCRs). Structurally, each β-adrenergic receptor is an integral membrane protein composed of seven hydrophobic α-helices that span the plasma membrane, earning it the name heptahelical receptor. Upon epinephrine binding to a site located deep within the receptor’s transmembrane domain, a conformational change is induced (Figure 1). This change alters the intracellular domain of the receptor. This promotes the exchange of GDP for GTP on the associated G-protein, thereby activating downstream signaling pathways.
Stimulatory G Protein
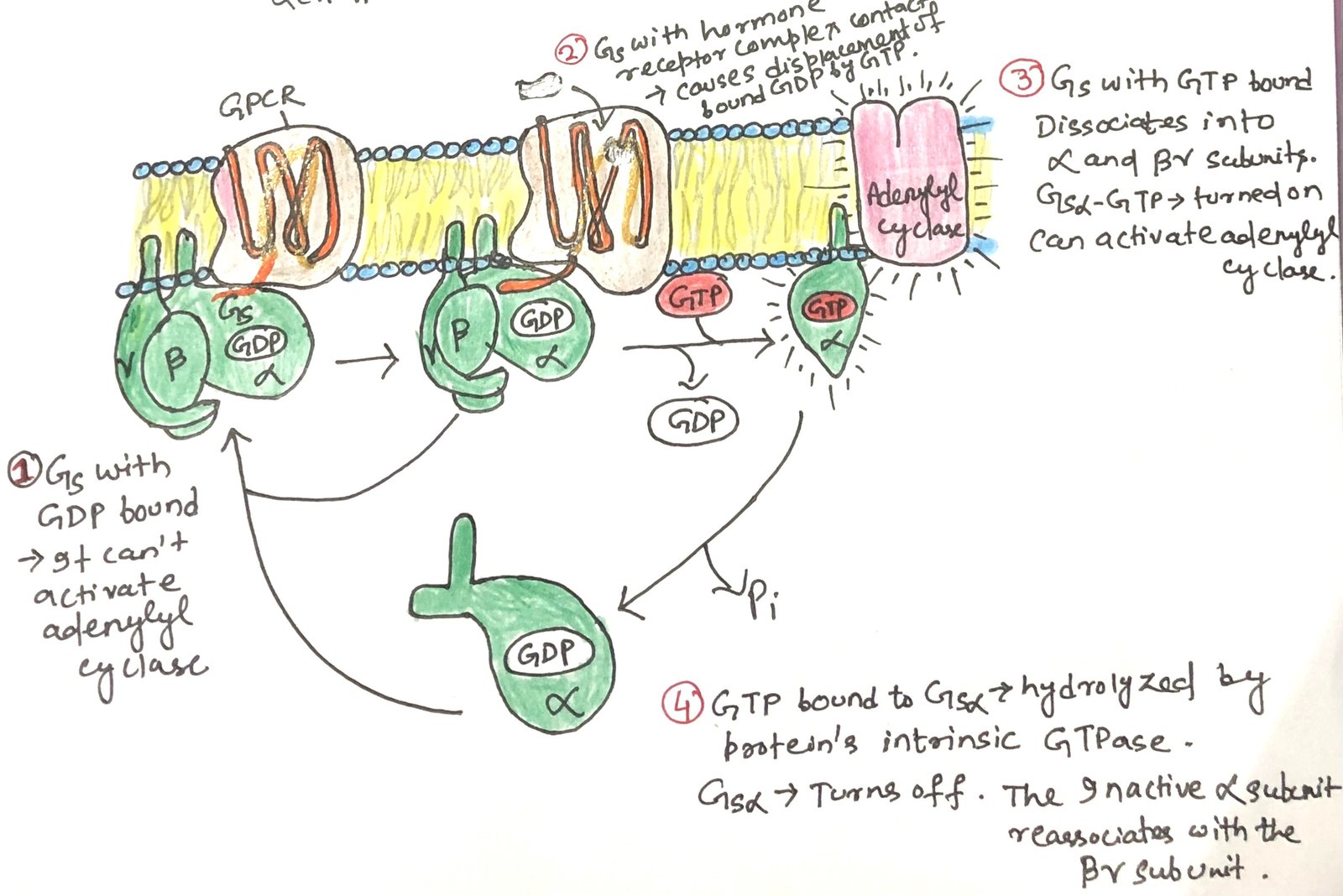
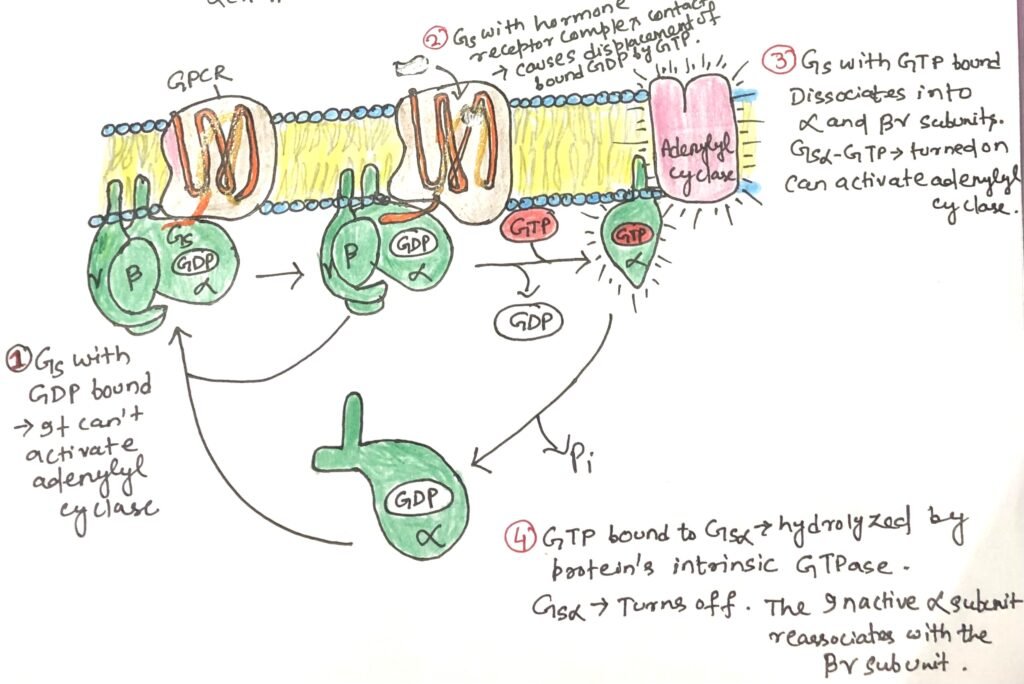
All G-protein coupled receptors (GPCRs) interact with heterotrimeric G proteins. The G proteins are made up of three different subunits: α, β, and γ. These are called trimeric G proteins. In this system, the αsubunit is responsible for binding GDP or GTP and for relaying signals from the activated receptor to the effector protein. When the G protein stimulates its target, it is known as a stimulatory G protein or GS.
GS acts like a molecular switch. When GTP is bound to its α subunit, GS is in the “on” state and can activate its effector. When GDP is bound, the G protein is “off.” Upon activation, the β and γ subunits remain together as a βγ dimer and separate from the α subunit. Then the activated α subunit GS carrying GTP travels along the membrane to stimulate a nearby enzyme, adenylyl cyclase.
Synthesis of a Second Messenger cAMP
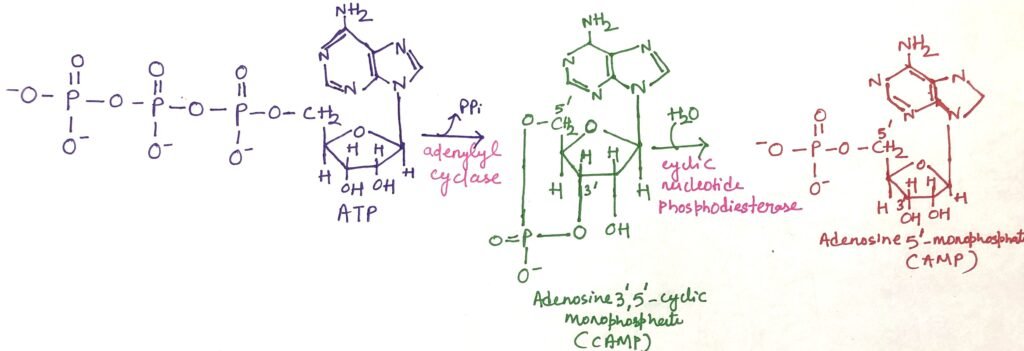
Adenylyl cyclase is an integral membrane protein with its active site facing the cytoplasm. When the active GSα subunit binds to adenylyl cyclase, it activates the enzyme, triggering the conversion of ATP into the second messenger cAMP. It therefore increases the concentration of cAMP in the cytosol (Figure 1). Hydrolysis of cAMP to adenosine 5’-monophosphate (AMP) is done by the enzyme cAMP phosphodiesterase (Figure 2).
The interaction between GSα and adenylyl cyclase is enabled by GSα being bound to GTP. The GSα subunit has a built-in GTPase activity, which allows it to turn itself off by hydrolyzing its bound GTP into GDP (Figure 3). Once GTP is converted to GDP, GSα becomes inactive and separates from adenylyl cyclase, causing the enzyme to stop functioning. The inactive GSα then recombines with the βγ dimer (GSβγ) to reform the inactive GS protein, which is now ready to respond again when a hormone binds to the receptor.
cAMP Activates PKA
Epinephrine initiates its downstream effects by increasing the intracellular concentration of cyclic AMP (cAMP) through the activation of adenylyl cyclase. The resulting cAMP, which acts as a second messenger, allosterically activates cAMP-dependent protein kinase, also known as protein kinase A (PKA). Activated PKA then phosphorylates specific serine (Ser) or threonine (Thr) residues on target proteins, including glycogen phosphorylase b kinase. When phosphorylated, this kinase becomes active and initiates the breakdown of glycogen stores in the muscle and liver. This prepares the body to meet increased energy demands as signaled by epinephrine.
cAMP-Mediated Activation Mechanism of PKA
The inactive form of protein kinase A (PKA) exists as a tetramer composed of two catalytic subunits (C) and two regulatory subunits (R), forming an R₂C₂ complex (Figure 4). This complex is catalytically inactive as each R subunit contains an autoinhibitory domain that blocks the substrate-binding site of the C subunits.
Cyclic AMP (cAMP) acts as an allosteric activator of PKA. When cAMP binds to the R subunits, it induces a conformational change that removes the autoinhibitory domain from the catalytic cleft. As a result, the R₂C₂ complex dissociates, releasing two free and active catalytic subunits (Figure 4).
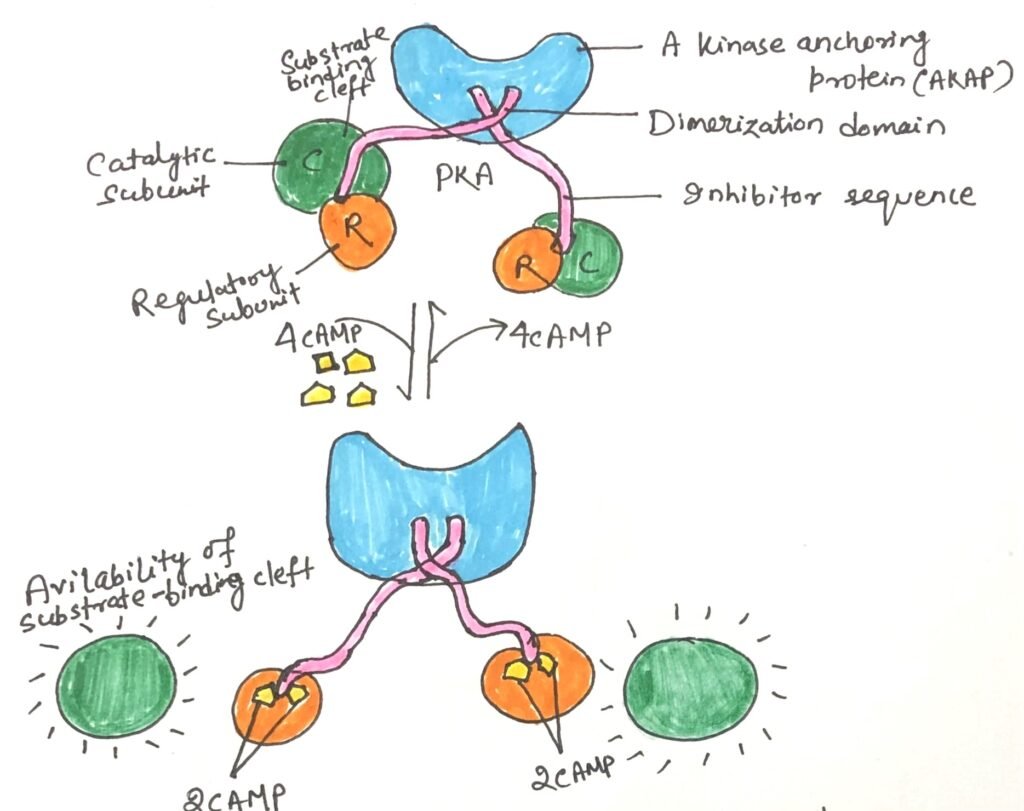
The substrate-binding cleft of PKA serves as the structural prototype for all known protein kinases. Key amino acid residues in this region are conserved across all 544 protein kinases encoded in the human genome. Each catalytic subunit has an ATP-binding site that positions ATP precisely. This enables the transfer of the terminal γ-phosphoryl group to the hydroxyl group of a serine or threonine residue on target proteins.
PKA Target Recognition Through Conserved Motifs
PKA regulates a wide range of enzymes further downstream in the signaling pathway. Although these target proteins have different functions, they all share a common sequence motif surrounding the serine (Ser) or threonine (Thr) residue that PKA phosphorylates. This specific sequence acts as a recognition signal, marking the protein as a substrate for PKA.
The substrate-binding cleft of PKA identifies and binds to this conserved sequence. This allows it to phosphorylate the Ser or Thr residue. By comparing multiple protein substrates of PKA, scientists have identified a consensus sequence. This is a common pattern of neighboring amino acids that determines whether a Ser or Thr can be targeted for phosphorylation by PKA.
Signal Amplification through Adenylyl Cyclase in the β-Adrenergic Pathway Involves Multiple Steps
Adenylyl cyclase plays a central role in a multi-step signal transduction cascade that significantly amplifies the original hormonal signal. When a single hormone molecule (such as epinephrine) binds to a β-adrenergic receptor, it catalytically activates multiple GS, one after another. Each activated GSα subunit then stimulates one adenylyl cyclase enzyme. This, in turn, produces many molecules of cyclic AMP (cAMP) (Figure 5).
The cAMP molecules act as second messengers, activating protein kinase A (PKA). Each active PKA molecule then phosphorylates numerous target proteins, such as phosphorylase b kinase. This subsequently activates glycogen phosphorylase b, an enzyme responsible for the release of glucose from glycogen stores (Figure 5).
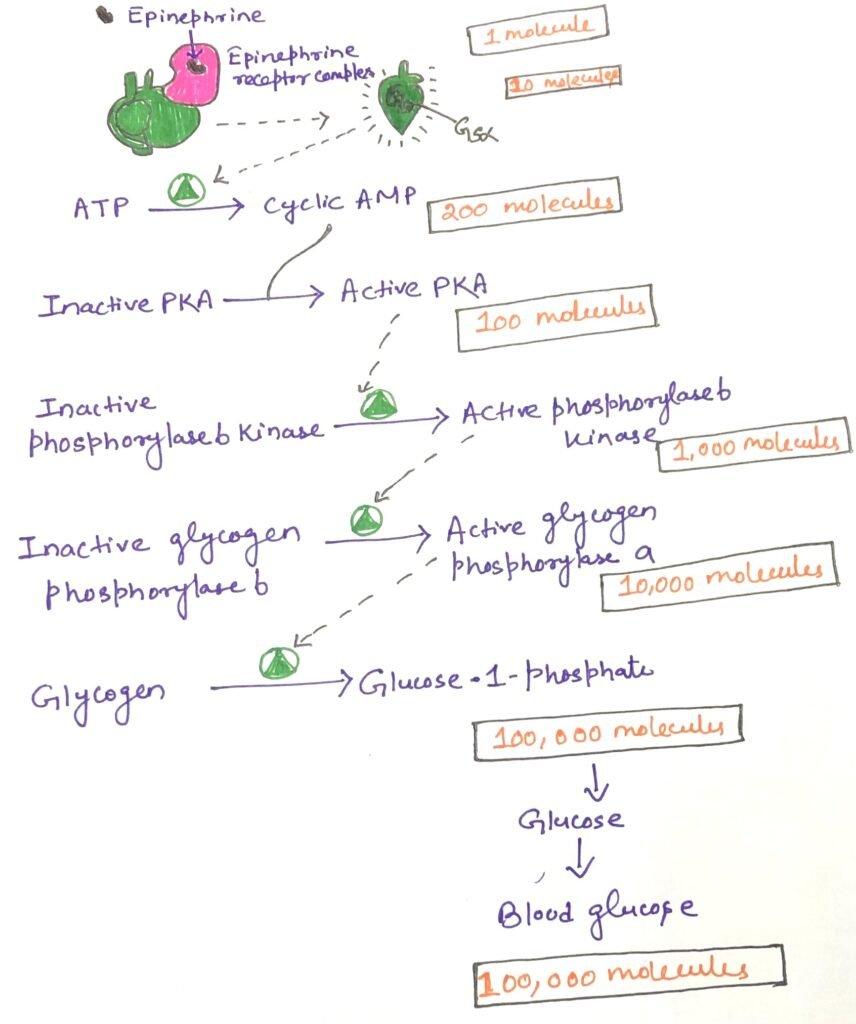
This cascade results in a massive amplification of the original signal. This enables a small amount of hormone to trigger a significant physiological response. Remarkably, this signaling pathway is extremely rapid, often producing intracellular effects within microseconds of hormone binding.
Conclusion
The β-adrenergic receptors are crucial in tissues such as skeletal muscle, liver, and adipose tissue. In these regions, they regulate various aspects of fuel metabolism in response to the hormone epinephrine. These receptors belong to the family of G-protein-coupled receptors (GPCRs). Structurally, each β-adrenergic receptor is an integral membrane protein composed of seven hydrophobic α-helices that span the plasma membrane, earning it the name heptahelical receptor.
All G-protein coupled receptors (GPCRs) interact with heterotrimeric G proteins, which are made up of three different subunits: α, β, and γ. These are called trimeric G proteins. When the G protein stimulates its target, it is known as a stimulatory G protein or GS. It acts like a molecular switch. When GTP is bound to its α subunit, GS is in the “on” state and can activate its effector. When GDP is bound, the G protein is “off.”
Adenylyl cyclase is an integral membrane protein with its active site facing the cytoplasm. When the active GSα subunit binds to adenylyl cyclase, it activates the enzyme, triggering the conversion of ATP into the second messenger cAMP, thereby increasing the concentration of cAMP in the cytosol. Cyclic AMP (cAMP) acts as an allosteric activator of PKA. PKA regulates a wide range of enzymes further downstream in the signaling pathway. Adenylyl cyclase plays a central role in a multi-step signal transduction cascade that significantly amplifies the original hormonal signal.
You may also like:
- Role of G protein-coupled receptors in vision
- Role of G Protein-Coupled Receptors in Vertebrate Olfaction and Gustation

I, Swagatika Sahu (author of this website), have done my master’s in Biotechnology. I have around fourteen years of experience in writing and believe that writing is a great way to share knowledge. I hope the articles on the website will help users in enhancing their intellect in Biotechnology.